Niels Bohr: Biography & Atomic Theory

Niels Bohr was one of the foremost scientists of modern physics, best known for his substantial contributions to quantum theory and his Nobel Prize-winning research on the structure of atoms.
Born in Copenhagen in 1885 to well-educated parents, Bohr became interested in physics at a young age. He studied the subject throughout his undergraduate and graduate years and earned a doctorate in physics in 1911 from Copenhagen University.
While still a student, Bohr won a contest put on by the Academy of Sciences in Copenhagen for his investigation into the measurements of liquid surface tension using oscillating fluid jets. Working in the laboratory of his father (a renowned physiologist), Bohr conducted several experiments and even made his own glass test tubes.
Bohr went above and beyond the current theory of liquid surface tension by taking into account the viscosity of the water as well as incorporating finite amplitudes rather than infinitesimal ones. He submitted his essay at the last minute, winning first place and a gold medal. He improved upon these ideas and sent them to the Royal Society in London, who published them in the journal Philosophical Transactions of the Royal Society in 1908, according to Nobelprize.org.
His subsequent work became increasingly theoretical. It was while conducting research for his doctoral thesis on the electron theory of metals that Bohr first came across Max Planck's early quantum theory, which described energy as tiny particles, or quanta.
In 1912, Bohr was working for the Nobel laureate J.J. Thompson in England when he was introduced to Ernest Rutherford, whose discovery of the nucleus and development of an atomic model had earned him a Nobel Prize in chemistry in 1908. Under Rutherford's tutelage, Bohr began studying the properties of atoms.
Bohr held a lectureship in physics at Copenhagen University from 1913 to 1914 and went on to hold a similar position at Victoria University in Manchester from 1914 to 1916. He went back to Copenhagen University in 1916 to become a professor of theoretical physics. In 1920, he was appointed the head of the Institute for Theoretical Physics.
Combining Rutherford's description of the nucleus and Planck's theory about quanta, Bohr explained what happens inside an atom and developed a picture of atomic structure. This work earned him a Nobel Prize of his own in 1922.
In the same year that he began his studies with Rutherford, Bohr married the love of his life, Margaret Nørlund, with whom he had six sons. Later in life, he became president of the Royal Danish Academy of Sciences, as well as a member of scientific academies all over the world.
When the Nazis invaded Denmark in World War II, Bohr managed to escape to Sweden. He spent the last two years of the war in England and the United States, where he got involved with the Atomic Energy Project. It was important to him, however, to use his skills for good and not violence. He dedicated his work toward the peaceful use of atomic physics and toward solving political problems arising from the development of atomic weapons of destruction. He believed that nations should be completely open with one another and wrote down these views in his Open Letter to the United Nations in 1950.
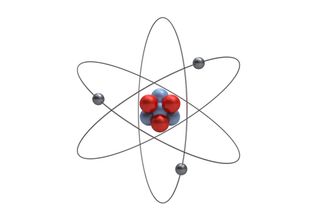
Atomic model
Bohr's greatest contribution to modern physics was the atomic model. The Bohr model shows the atom as a small, positively charged nucleus surrounded by orbiting electrons.
Bohr was the first to discover that electrons travel in separate orbits around the nucleus and that the number of electrons in the outer orbit determines the properties of an element.
The chemical element bohrium (Bh), No. 107 on the periodic table of elements, is named for him.
Liquid droplet theory
Bohr's theoretical work contributed significantly to scientists' understanding of nuclear fission. According to his liquid droplet theory, a liquid drop provides an accurate representation of an atom's nucleus.
This theory was instrumental in the first attempts to split uranium atoms in the 1930s, an important step in the development of the atomic bomb.
Despite his contributions to the U.S. Atomic Energy Project during World War II, Bohr was an outspoken advocate for the peaceful application of atomic physics.
Quantum theory
Bohr's concept of complementarity, which he wrote about in a number of essays between 1933 and 1962, states that an electron can be viewed in two ways, either as a particle or as a wave, but never both at the same time.
This concept, which forms the basis of early quantum theory, also explains that regardless of how one views an electron, all understanding of its properties must be rooted in empirical measurement. Bohr's theory stresses the point that an experiment's results are deeply affected by the measurement tools used to carry them out.
Bohr's contributions to the study of quantum mechanics are forever memorialized at the Institute for Theoretical Physics at Copenhagen University, which he helped found in 1920 and headed until his death in 1962. It has since been renamed the Niels Bohr Institute in his honor.
Niels Bohr quotations
"Every great and deep difficulty bears in itself its own solution. It forces us to change our thinking in order to find it."
"Everything we call real is made of things that cannot be regarded as real."
"The best weapon of a dictatorship is secrecy, but the best weapon of a democracy should be the weapon of openness."
"Never express yourself more clearly than you are able to think."
Additional reporting by Traci Pedersen, Live Science contributor
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

Elizabeth is a former Live Science associate editor and current director of audience development at the Chamber of Commerce. She graduated with a bachelor of arts degree from George Washington University. Elizabeth has traveled throughout the Americas, studying political systems and indigenous cultures and teaching English to students of all ages.