Quantum Particles Take the Road Most Traveled
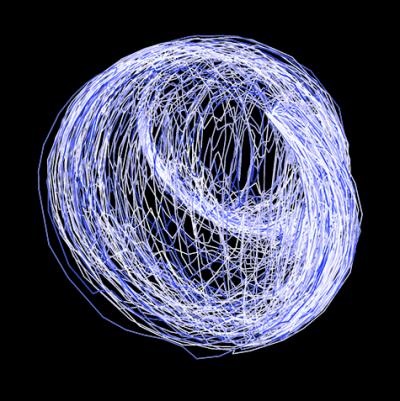
For the first time ever, physicists have mapped the path that particles are most likely to take when moving from one quantum state to another.
In physics, a concept called the "path of least action" describes the trajectory that an object is most likely to follow, similar to the familiar concept of the "path of least resistance." For example, a tossed football follows a parabolic arc through the air instead of spinning off in crazy loops or zigzags. That's because a parabola path requires fewer "actions" than a looped or zigzag path.
However, physicists didn't know whether quantum particles, like electrons, neutrinos or photons, follow the same rule. Many of the classic rules of physics don't seem to apply to these tiny particles. Instead, they are governed by the weird rules of quantum mechanics that even Einstein called "spooky." [Wacky Physics: The Coolest Little Particles in Nature]
Quantum particles can exist in states where they are in multiple places at once — a phenomenon called superposition. A mathematical equation called a wave function describes the many possible locations where a quantum particle might simultaneously exist. But as soon as someone tries to measure the location or the velocity of one of these particles, its wave function collapses and the particle will appear in only one spot, falling back under the laws of conventional physics.
This makes studying quantum particles extremely difficult, because the moment scientists start probing around, the particles' quantum states collapse. However, physicists have developed a way to isolate the wacky quantum world and peer into it in a noninvasive way; this allows them to map the path that particles are most likely to take when changing from one state to another.
"It's a great breakthrough in terms of being able to monitor quantum systems,"Andrew Jordan, a physicist at the University of Rochester, who worked on the original theory, told Live Science. "We're just scratching the surface of the kinds of physics permitted here."
Jordan developed the theory, and brought the idea to experimental physicists at the University of California, Berkeley and Washington University in St. Louis who helped design an experiment to test it. Kater Murch, a professor of physics at Washington University, sketched out possible paths that the particles might take, then polled the research team to see which path they thought the experiment would most likely reveal.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"We're all experts, but no one agreed,"Murch told Live Science. "We had no idea how one quantum state gets to another."
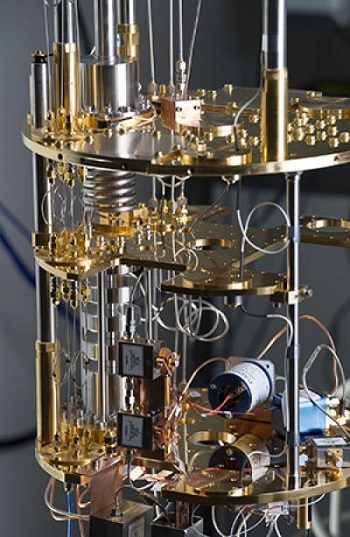
The team used a superconducting quantum device, essentially a circuit inside a box made out of copper, Murch explained. The system is modeled after an atom. It has multiple quantum energy levels just like an atom, and it's sometimes referred to as an "artificial atom," Murch said.
The researchers beamed a stream of microwave particles into the box. These particles interacted with the superconducting circuit and then reflected back out. Along the way, the particles ended up in either a ground state (the lowest energy state) or an excited state (any state with a higher energy level than the ground state). An infinite number of superpositions exist between these two states, so the researchers repeated the experiment 1 million times to determine the most commonly occurring path.
The results revealed that the particles most frequently travel a convex curve. The equation is simple, and it's fairly easy to calculate the path the particles are most likely to take, Jordan said.
Murch said the results of the experiment could be a step toward the "holy grail"of chemistry – maximizing the efficiency of chemical reactions.
"At its most basic level, a chemical reaction changes quantum states from one to another," Murch said. "Understanding that route could help chemists produce more efficient chemical reactions."
The research could also one day lead to a way for physicists to directly control quantum systems, Jordan said.
Details of the experiment were published in the July 31 issue of the journal Nature.
Follow Kelly Dickerson on Twitter. Follow us @livescience, Facebook & Google+. Original article on Live Science.











