COVID-19 vaccines: The new technology that made them possible
The COVID-19 pandemic served as an unexpected proof of concept for mRNA vaccines.
Days before her 91st birthday, Margaret Keenan became the first person in the world to receive the Pfizer-BioNTech COVID-19 vaccine outside of clinical trials.
Keenan, who was sporting a polka-dot cardigan over a festive shirt, was given the first dose of a two-dose vaccine at the University Hospital Coventry in England, setting off the first mass vaccination effort against a virus that has now infected at least 70 million people worldwide and killed 1.5 million. An 81-year-old named William Shakespeare was next in line for the vaccine.
Keenan and Shakespeare are also the first humans, outside of a trial setting, to be given a vaccine that harnesses "mRNA" technology. This relatively new tech, which relies on a synthetic strand of genetic code called messenger RNA (mRNA) to prime the immune system, had not yet been approved for any previous vaccine in the world.
Related: Coronavirus live updates
But the COVID-19 pandemic served as an unexpected proof of concept for mRNA vaccines, which, experts told Live Science, have the potential to dramatically reshape vaccine production in the future. In fact, two COVID-19 vaccines developed by Pfizer and Moderna, are 95% and 94.1% effective, respectively, at preventing an infection with the novel coronavirus causing COVID-19.

On Thursday (Dec. 10), a panel of experts voted and recommended that the Food and Drug Administration (FDA) grant emergency approval to Pfizer's vaccine, or permission for it to be distributed prior to full approval under emergency situations like a pandemic. The panel is set to assess Moderna's vaccine on Dec. 17. Healthcare workers and vulnerable individuals in the U.S. could receive the Pfizer vaccine as early as next week.
COVID-19 has really "laid the foundation" for rapid production of new vaccines, such as mRNA vaccines, to fight future pathogens, said Maitreyi Shivkumar, a virologist and senior lecturer in molecular biology at De Montfort University in Leicester, England. "With the technology that we've developed for SARS-CoV-2, we can very easily transfer that to other emerging pathogens."
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Here's how mRNA vaccines work, and why they could make such a difference for vaccine development.
Tapping into a natural process
mRNA vaccines are inspired by basic biology.
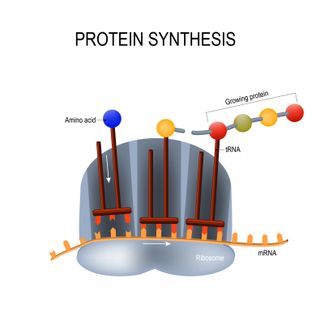
Cells store DNA that holds coded instructions for making proteins. When a cell needs to make a protein, it copies the appropriate instructions onto a messenger RNA molecule — a single strand of genetic material. A cellular machine called a ribosome then runs along this code, reads it, and shoots out the correct building blocks to make the protein. Proteins are the essential workers of the body, forming the structure of cells, making tissue, fueling chemical reactions and sending messages: Without them, everything would shut down.
Around three decades ago, scientists realized that they could synthesize mRNA in the lab, deliver it into human cells and use the body to make any protein they wanted, such as proteins that could help fight a range of diseases in the body from cancers to respiratory illnesses. In 1990, researchers at the University of Wisconsin and biotech company Vical Incorporated figured out how to make mRNA that could direct mice cells to create proteins, according to Business Insider.
In the 1990s, Hungarian-born scientist Katalin Karikó started building on this work, but ran into major roadblocks, the biggest being that the mice's immune system would deem synthetic mRNA foreign and destroy it, sometimes even creating a dangerous inflammatory response. A decade later, while working at the University of Pennsylvania, Karikó and her collaborator Dr. Drew Weissman, figured out that they could create an invisibility cloak for synthetic mRNA by swapping out a piece of the mRNA code for a slightly altered one, according to STAT News. That tiny edit allowed synthetic mRNA to slip right into cells without rousing the immune system, a finding that the researchers published in multiple papers starting in 2005, according to STAT News. These results caught the attention of two key scientists: one who later helped found Moderna and another who helped found BioNTech.
Neither company initially set out to develop mRNA vaccines against infectious diseases, but eventually started to expand into that field with mRNA flu, cytomegalovirus and Zika virus vaccines in development or clinical trials. But then a deadly virus presented a unique opportunity to test, in large groups of people, just how powerful the technology could be.
On Jan. 10, Chinese researchers first published the genetic sequence of the novel coronavirus on a preprint online; within a week, Weissman and his team at the University of Pennsylvania were already developing synthetic mRNA against the virus and both Moderna and Pfizer licensed this team's formulation from The University of Pennsylvania, according to a perspective posted on Sep. 3 in the journal JAMA.
Within 66 days of the sequence being published, Moderna, in collaboration with the National Institute of Allergy and Infectious Diseases, developed a vaccine and kickstarted the first U.S. clinical trial to test it against COVID-19.
Five of the vaccines currently in clinical trials are mRNA vaccines; though they are made from different recipes, they use the same underlying concept.
Both Moderna's and Pfizer's vaccines are made up of synthetic mRNA that carries the code for the spike protein. The mRNA is enveloped inside a fatty nanoparticle that acts as a Trojan horse, infiltrating human cells and delivering the spike-building instructions without awakening the immune system. Once cells have gotten hold of the mRNA, they create the spike protein, which in turn triggers the immune system to produce an arsenal of cells to fight the spike protein and thus protect the body against SARS-CoV-2.
'Mimicking a viral infection'
The vaccines developed by Moderna and Pfizer are likely so successful because they're "mimicking a viral infection," by activating two major immune responses in the body, said Dr. Otto Yang, a professor of medicine in the division of infectious diseases and of microbiology, immunology, and molecular genetics at the University of California, Los Angeles.
The better-known response involves antibodies: The cells expel the spike proteins they make; these trigger the immune system to create antibodies against them, Yang told Live Science. Antibodies are found in blood, tissues and fluids — but they can't access a virus that's already inside the cell, "so the immune system evolved a way to deal with that," Yang said.
Related: 14 coronavirus myths busted by science
That response involves killer T cells, also known as CD8 T cells. These killers scan cell surfaces — cells display small pieces of all the proteins they make on their surface — and destroy the ones that are infected by a virus. SARS-CoV-2 vaccines can also wave a warning flag for killer T cells: after the mRNA prompts cells to make the spike protein, cells display processed fragments of it on its surface.
This gives mRNA vaccines an advantage over more traditional vaccines such as those for flu or rabies, that are made from killed versions of the actual pathogen or their target proteins. Killed virus vaccines can't get into cells, so they trigger antibodies but not the killer T-cell response, Yang said.
But mRNA vaccines aren't the only ones that trigger both these immune responses; the University of Oxford vaccine, made from a weakened cold virus called an adenovirus that infects chimpanzees, also does, Yang said. This adenovirus is genetically modified to not be able to replicate in the body and to include the genetic code for the spike protein. These vaccines also prompt the cells to create the proteins themselves, rather than providing already-made ones; and because the cells make the proteins, they display fragments of them on their surfaces.
Vaccines like the Oxford vaccine also show great promise in the future of vaccine development, experts told Live Science. And such vector vaccines have been studied extensively when compared to mRNA vaccines, according to the JAMA perspective. But the Oxford vaccine, developed with AstraZeneca, showed less efficacy than the mRNA vaccines did; in late-stage clinical trials, the Oxford vaccine was 62% effective at protecting against COVID-19 in participants who were given two full doses and 90% effective at protecting those who were first given a half dose and then a full dose, according to findings published on Dec. 8 in the journal The Lancet.
It's not yet clear why, but one major possibility is that the Oxford vaccine could be overwhelming the immune system when people are given an initial full dose. In addition to the spike protein, the adenovirus also has its own proteins. Because all of these proteins are foreign to the body, the immune system creates defenses against all of them. "There's no way that the immune system has any sort of guidance that 'OK, I'm only supposed to make a response against spike,'" Yang said. On the other hand, the mRNA vaccines are more targeted, telling the immune system to respond only to the spike protein.
But before we can say that mRNA vaccines are fundamentally better than other options, Yang said, scientists need to see detailed data from the trials, rather than gleaning information from "snippets from press releases." It's also not yet known how long mRNA vaccine-induced immune responses will last. That being said, mRNA vaccines are the "first technology that allows us to [make killer T cell responses] without giving a whole live virus," Yang said. Though rare, live but weakened virus vaccines have a slight risk of causing a more serious disease, whereas mRNA vaccines, as far as we know, do not, he added.
mRNA vaccines do not integrate into our DNA (the DNA is stored in a cell's inner core called the nucleus, a place that the synthetic mRNA doesn't go) and the mRNA generally degrades after a few days, Shivkumar said.
In the first day that Pfizer's vaccine was administered to several thousand people, two people who had a history of severe allergic reactions had anaphylaxis-like symptoms, prompting the U.K.'s regulatory agency to warn people with severe allergies to avoid getting that particular vaccine. But experts say the general population shouldn't be anxious about getting this vaccine and it isn't totally unexpected as allergic reactions can occur with a number of vaccines, Live Science reported.
"I do not believe that mRNA vaccines pose any significant greater chance of a severe allergic reaction than other vaccines," said Justin Richner, an assistant professor in the department of microbiology and immunology at the University of Illinois (who previously, as a postdoctoral fellow, collaborated with Moderna on their as-of-yet unapproved mRNA vaccine to fight the Zika virus), noting that the safety data from the mRNA vaccine trials looked very similar to other vaccines. "If anything, I would predict that there is less likely to be an allergic reaction in the mRNA vaccines as the production does not require eggs like other vaccines," he said. (Most flu-vaccines are made using eggs so they can contain bits of egg protein, according to the CDC).
Swap the code
Another huge advantage of mRNA vaccines is how quickly and easily they can be developed.
"The beauty of the mRNA platform is that you can easily swap out the genetic code," Richner said. In theory, if scientists know what proteins to target on a virus to stop it from infecting human cells, such as the spike protein for SARS-CoV-2, they can use the same platform that was developed for other vaccines such as the COVID-19 vaccine and just swap out the code for the spike protein with the code for the new protein.
The real problem lies in finding the correct target, Richner said.
Because scientists had previously conducted research on similar coronaviruses — those that caused severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) — they knew early on that the spike protein was probably the optimal target, Richner said. But they may not get so lucky with other viruses, as previous failures, such as with HIV, have revealed.
mRNA vaccines are cheaper, easier and faster to develop, and in theory, scale up more easily when compared to traditional vaccines. That's because older vaccine technologies rely on growing the virus or the proteins in the lab, Shivkumar said. Traditional vaccines are often grown in eggs or cells and then weakened or killed.
"After obtaining knowledge of the pathogen," researchers can synthesize and have an mRNA vaccine ready for delivery in about a week, Richner said. "For traditional vaccine development, this process would take at a minimum one month and usually several months."
mRNA is a chemical that can be made in a factory setting (such as in a test tube or tank) "relatively easily" once a pipeline is established, Richner said. "Manufacturing is going to be a big advantage going forward." Pfizer recently experienced production delays, but those delays are only "because it's the first time making an mRNA vaccine to this scale," he added.
Still, this easy genetic swap isn't a sole capability of mRNA vaccines, as the adenovirus vector vaccines also have this advantage. "The Oxford vaccine is more traditional, but it is, again, slightly sort of a jump from the traditional ones because it has the same backbone," Shivkumar said. Prior to the pandemic, the group that was developing the Oxford vaccine was working on a vaccine against the coronavirus that causes MERS so "they actually just swapped in the SARS-CoV-2 sequence into that same backbone," she said. But with the adenovirus vector vaccines, scientists still have to rely on the slower biological processes, namely, growing an adenovirus in the lab.
Theoretically, mRNA vaccines can tackle any virus — and one day, might even be able to tackle multiple pathogens at once, according to the JAMA perspective. But practically, we won't know how universal these vaccines can become when confronted with a variety of new viruses. SARS-CoV-2 is "not a particularly difficult virus," said Dennis Burton, a professor of immunology and microbiology at the Scripps Research Institute in California. There will likely be "more severe tests and then you'll be better able to judge how universal RNA vaccines could be. Still, there's "every chance" mRNA can be truly revolutionary, but we need more information before we can be sure, he told Live Science.
Either way, no matter how quickly genetic information can be swapped in and out of mRNA vaccines, "you can't skip all the safety data," Richner said. The "slowdown is always going to be the clinical trial," Richner said.
Polar temperatures

Despite their promise, mRNA vaccines still have some limitations. For instance, right now, Pfizer's mrNA vaccine must be stored at polar temperatures of minus 94 degrees Fahrenheit (minus 70 degrees Celsius). "Especially in developing countries and countries where it's impossible to have minus-80 freezers everywhere, I think it's still not ideal, so you would still need to rely on the more traditional vaccines," Shivkumar said.
Moderna's vaccine can be stored at freezer temperatures of minus 4 F (minus 20 C). The difference in storage requirements between the two vaccines likely comes down to the recipe that the company used to make them; the ultra-cold temperature may keep either the nanoparticle shell or the mRNA more stable, Yang said.
But if those mRNA vaccines could be stored and delivered at higher temperatures, with the impressive efficacy that they showed, "I can imagine that they will sort of be a game-changer globally," Shivkumar added. In the future, Pfizer may be able to improve their vaccine to be more stable at higher temperatures, Richner said.
Related: The most promising coronavirus vaccine candidates
In the past, mRNA vaccines didn't produce a strong enough response compared to more traditional vaccines, Shivkumar said. "Because with the mRNA you use such low levels and it degrades so quickly, the amount of protein produced will be relatively lower than if you were to be given either a protein or an attenuated virus," she said. But clearly scientists have figured out how to make mRNA stable enough to trigger a strong protective response. While this would need to be checked with every pathogen, it's clear the technology has "definitely improved," Shivkumar added.
"It's very exciting to have these mRNA vaccines," said Dr. Octavio Ramilo, the chief of infectious diseases at Nationwide Children's Hospital, Columbus, Ohio. But "it will be good to have more than just one strategy," because you never know which one will stick, he said. Not all platforms will necessarily work as well for every pathogen, especially since each virus might have a unique strategy to hide from the immune system, he added.
It's also important to understand how these vaccines will work in children and the elderly, Ramilo said. Though many of the trials have included elderly participants, children have been absent. Children can respond to vaccines differently than adults, Ramilo told Live Science.
Especially babies' immune systems change "dramatically in the first year," Ramilo said. The flu virus tends to impact children and the elderly more severely than other age groups, Ramilo said. But vaccines don't work as well in those groups, he said. So having multiple platforms and understanding how they work "is going to be fundamental to leverage and to make them work in different situations," he added.
And if another new virus comes along years from now, we'll hopefully have learned lessons from 2020. The pandemic served as a "proof of concept" that mRNA experts had been waiting for, Yang said. The fastest vaccine developed to date prior to the COVID-19 pandemic was the Mumps vaccine, which took four years to develop and license it in 1967. Not counting the years it took to develop the vaccine, the Ebola vaccine was the fastest ever tested in clinical trials — which took less than a year — during the Ebola outbreak across West Africa. That is, until the world was faced with a deadly pandemic.
Just nine months into the pandemic, the fact that new vaccines are already finished clinical trials "is pretty impressive," Yang said. "When you're talking about a vaccine possibly being FDA-approved only a few months after it was first tried in a human that is amazingly fast."
"I'm not sure that it could actually be much quicker than this."
Originally published on Live Science.

Yasemin is a staff writer at Live Science, covering health, neuroscience and biology. Her work has appeared in Scientific American, Science and the San Jose Mercury News. She has a bachelor's degree in biomedical engineering from the University of Connecticut and a graduate certificate in science communication from the University of California, Santa Cruz.