Powerful DNA 'Editing' Has Arrived, Are We Ready for It?
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
This article was originally published on The Conversation. The publication contributed this article to Live Science's Expert Voices: Op-Ed & Insights.
CRISPR/Cas is a new technology that allows unprecedented control over the DNA code. It’s sparked a revolution in the fields of genetics and cell biology, becoming the scientific equivalent of a household name by raising hopes about new ways to cure diseases including cancer and to unlock the remaining mysteries of our cells.
The gene editing technique also raises concerns. Could the new tools allow parents to order “designer babies”? Could premature use in patients lead to unforeseen and potentially dangerous consequences? This potential for abuse or misuse led prominent scientists to call for a halt on some types of new research until ethical issues can be discussed – a voluntary ban that was swiftly ignored in some quarters.
The moratorium is a positive step toward preserving the public’s trust and safety, while the promising new technology can be further studied.
Editing DNA to cure disease
While most human diseases are caused, at least partially, by mutations in our DNA, current therapies treat the symptoms of these mutations but not the genetic root cause. For example, cystic fibrosis, which causes the lungs to fill with excess mucus, is caused by a single DNA mutation. However, cystic fibrosis treatments focus on the symptoms – working to reduce mucus in the lungs and fight off infections – rather than correcting the mutation itself. That’s because making precise changes to the three-billion-letter DNA code remains a challenge even in a Petri dish, and it is unprecedented in living patients. (The only current example of gene therapy, called Glybera, does not involve modifying the patient’s DNA, and has been approved for limited use in Europe to treat patients with a digestive disorder.)
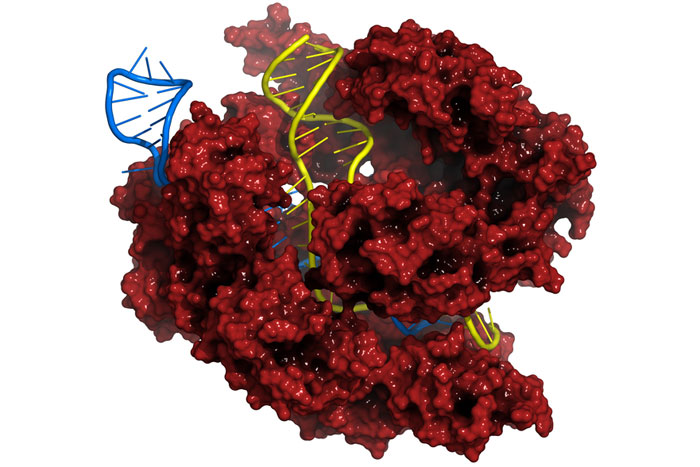
That all changed in 2012, when several research groups demonstrated that a DNA-cutting technology called CRISPR/Cas could operate on human DNA. Compared to previous, inefficient methods for editing DNA, CRISPR/Cas offers a shortcut. It acts like a pair of DNA scissors that cut where prompted by a special strand of RNA (a close chemical relative of DNA). Snipping DNA turns on the cell’s DNA repair process, which can be hijacked to either disable a gene – say, one that allows tumor cells to grow uncontrollably – or to fix a broken gene, such as the mutation that causes cystic fibrosis. The advantages of the Cas9 system over its predecessor genome-editing technologies – its high specificity and the ease of navigating to a specific DNA sequence with the “guide RNA” – have contributed to its rapid adoption in the scientific community.
The barrier to fixing the DNA of diseased cells appears to have evaporated.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Playing with fire
With the advance of this technique, the obstacles to altering genes in embryos are falling away, opening the door to so-called “designer babies” with altered appearance or intelligence. Ethicists have long feared the consequences of allowing parents to choose the traits of their babies. Further, there is a wide gap between our understanding of disease and the genes that might cause them. Even if we were capable of performing flawless genetic surgery, we don’t yet know how specific changes to the DNA will manifest in a living human. Finally, the editing of germ line cells such as embryos could permanently introduce altered DNA into the gene pool to be inherited by descendants.
And making cuts in one’s DNA is not without risks. Cas9 – the scissor protein – is known to cleave DNA at unintended or “off-target” sites in the genome. Were Cas9 to inappropriately chop an important gene and inactivate it, the therapy could cause cancer instead of curing it.
Take it slow
All the concerns around Cas9 triggered a very unusual event: a call from prominent scientists to halt some of this research. In March of 2015, a group of researchers and lawyers called for a voluntary pause on further using CRISPR technology in germ line cells until ethical guidelines could be decided.
Writing in the journal Science, the group – including two Nobel laureates and the inventors of the CRISPR technology – noted that we don’t yet understand enough about the link between our health and our DNA sequence. Even if a perfectly accurate DNA-editing system existed – and Cas9 surely doesn’t yet qualify – it would still be premature to treat patients with genetic surgery. The authors disavowed genome editing only in specific cell types such as embryos, while encouraging the basic research that would put future therapeutic editing on a firmer foundation of evidence.
Pushing ahead
Despite this call for CRISPR/Cas research to be halted, a Chinese research group reported on their attempts at editing human embryos only two months later. Described in the journal Protein & Cell, the authors treated nonviable embryos to fix a gene mutation that causes a blood disease called β-thalassemia.
The study results proved the concerns of the Science group to be well-founded. The treatment killed nearly one in five embryos, and only half of the surviving cells had their DNA modified. Of the cells that were even modified, only a fraction had the disease mutation repaired. The study also revealed off-target DNA cutting and incomplete editing among all the cells of a single embryo. Obviously these kinds of errors are problematic in embryos meant to mature into fully grown human beings.
George Daley, a Harvard biologist and member of the group that called for the moratorium, concluded that “their study should be a stern warning to any practitioner who thinks the technology is ready for testing to eradicate disease genes."
In the enthusiasm and hype surrounding Cas9, it is easy to forget that the technology has been in wide use for barely three years.
Role of a moratorium
Despite the publication of the Protein & Cell study – whose experiments likely took place at least months earlier – the Science plea for a moratorium can already be considered a success. The request from such a respected group has brought visibility to the topic and put pressure on universities, regulatory boards and the editors of scientific journals to discourage such research. (As evidence of this pressure, the Chinese authors were rejected from at least two top science journals before getting their paper accepted.) And the response to the voluntary ban has thus far not included accusations of “stifling academic freedom,” possibly due to the scientific credibility of the organizers.
While rare, the call for a moratorium on research for ethical reasons can be traced to an earlier controversy over DNA technology. In 1975, a group that came to be known as the Asilomar Conference called for caution with an emerging technology called recombinant DNA until its safety could be evaluated and ethical guidelines could be published. The similarity between the two approaches is no coincidence: several authors of the Science essay were also members of the Asilomar team.
The Asilomar guidelines are now widely viewed as having been a proportionate and responsible measure, placing the right emphasis on safety and ethics without hampering research progress. It turns out recombinant DNA technology was much less dangerous than originally feared; existing evidence already shows that we might not be so lucky with Cas9. Another important legacy of the Asilomar conference was the promotion of an open discussion involving experts as well as the general public. By heeding the lessons of caution and public engagement, hopefully the saga of CRISPR/Cas will unfold in a similarly responsible – yet exciting – way.
Jeff Bessen is PhD Candidate in Chemical Biology at Harvard University.
This article was originally published on The Conversation. Read the original article. Follow all of the Expert Voices issues and debates — and become part of the discussion — on Facebook, Twitter and Google +. The views expressed are those of the author and do not necessarily reflect the views of the publisher. This version of the article was originally published onLiveScience.
 Live Science Plus
Live Science Plus