Bee Pollen Could Boost Battery Performance

Pollen — the pesky, sneeze-inducing stuff that makes allergy sufferers everywhere miserable — could be the next greatest thing in battery research, according to a new study.
Scientists at Purdue University, in West Lafayette, Indiana, have been researching ways to make better batteries, and recently discovered that pollen grains, and their unique microstructures, could be put to use as a more efficient type of energy storage unit.
Batteries are made up of three main parts: electrodes, an electrolyte and a separator. Each battery has two electrodes. One is the cathode, which is the positively charged end of the battery. The other is the anode, or the negatively charged end of the battery. The electrolyte runs through the anode and the cathode, divided by a separator, to create a current of electricity. [Inside Look at How Batteries Work (Infographic)]
The scientists were trying to improve on conventional lithium-ion batteries, which are the types of batteries typically used in cellphones and laptops. A lithium-ion battery has an anode made of carbon — usually graphite — and a cathode made of lithium cobalt oxide. The electrolyte that runs through the battery is made of lithium salts, said Vilas Pol, lead author of the new study and an associate professor in the School of Chemical Engineering and the School of Materials Engineering at Purdue University.
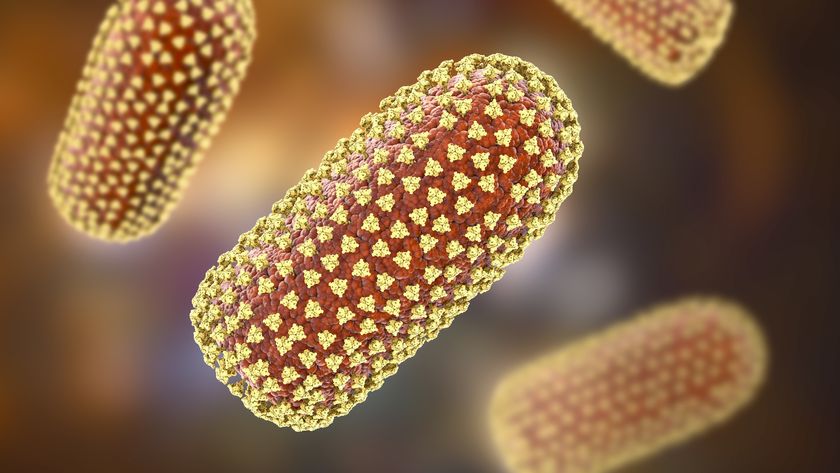
The researchers found that if they could turn pollen into a carbon anode with a more useful microstructure than graphite, they might be able to create a battery with the ability to store more energy. The scientists took pollen from honeybees and pollen from cattails, a common plant found near many bodies of water in North America, and turned them into little pieces of carbon. They did this by superheating a section of bee pollen and a section of cattail pollen to 1,112 degrees Fahrenheit (600 degrees Celsius) in a space that was filled with argon gas, which stops the carbon from burning up like it would if it was just heated by itself in a conventional oven, Pol said.
The scientists then reheated the pollen-based carbon pieces to create more empty pockets in the pollen structure, which increases their capacity to store energy, Pol said.
The researchers tested both types of pollen-based carbons in lithium-ion batteries and found that cattail pollen-based carbon had more energy-storing capacity than the bee pollen-based carbon, according to the study. This could be because cattail pollen has a more uniform structure, since it is made up of only one kind of pollen, the scientists said. Bee pollen, on the other hand, comes from the many different plants visited by honeybees and has a more irregular structure.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Next, Pol and his colleagues are planning to investigate how to create a better cathode (to go along with the new anode) to further improve a battery's energy storage.
"This is just the beginning of better batteries," Pol said.
The research was published online Feb. 5 in the journal Scientific Reports.
Follow Elizabeth Newbern @liznewbern. Follow Live Science @livescience, Facebook & Google+. Original article on Live Science.