Mini-Brains Allow Scientists to Study Brain Disorders
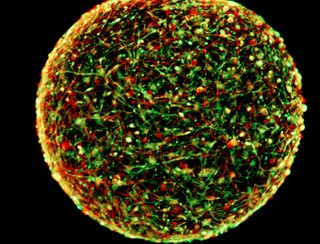
WASHINGTON — This is your bedbug-size brain on drugs. Researchers at Johns Hopkins University in Baltimore are growing "mini-brains" — smaller than the period at the end of this sentence — that may contain enough human brain cells to be useful in studying drug addiction and other neurological diseases.
The mini-brains, grown in a laboratory dish, could one day reduce the need for the use of laboratory animals to conduct this type of research or to test therapeutic drugs, the researchers said.
Labs from around the world have been racing to grow these and other organoids — microscopic, yet primitively functional versions of livers, kidneys, hearts and brains grown from real human cells. The version of the mini-brain from Johns Hopkins represents an advance over others reported in the last three years, in that it is quickly reproducible and contains many types of brain cells that interact with each other, just like a real brain, the researchers said.
The researchers, led by Dr. Thomas Hartung, director of the Johns Hopkins Center for Alternatives to Animal Testing, reported their progress on Feb. 13 at the annual meeting of the American Association for the Advancement of Science. [11 Body Parts Grown in the Lab]
Hartung noted that the mini-brain cannot yet replace animal models in the study of neurological diseases. But he added that the concept, which until quite recently seemed years from maturity, may be realized in as little as 10 months.
Growing organoids involves the use of cells called induced pluripotent stem (iPS) cells, a technology developed by Japanese researcher Shinya Yamanaka, who won the Nobel Prize in 2012 for that line of research. With iPS cell technology, scientists can theoretically turn back the clock in any type of mature cell — be it skin, muscle, bone, etc. — and bring it to a near-embryonic state. From there, cells can be coaxed into developing into any of a number of cell types, much in the same way that actual human embryonic cells develop into all the cell types that make up the human body.
Several labs are growing mini-brains. The first researchers to accomplish this, in 2013, were Jüergen Knoblich of the Institute of Molecular Biotechnology in Vienna, Austria, and Madeline Lancaster of the MRC Laboratory of Molecular Biology in Cambridge, England.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
These researchers said they can grow globular mini-brains a few millimeters in diameter in about three months, and that these organoids may be ideal for the study of fetal brain development, including microcephaly, the incomplete brain growth seen in some infants that researchers say may be linked with the Zika virus.
Hartung's group has taken a different approach to grow smaller mini-brains, about 350 microns (0.35 millimeters) across, but say their method has easier reproducibility, a greater diversity of brain cell types and takes less time — only 10 weeks.
He described them as "Mini Coopers" in that they are small but identical, ideal for comparative studies, as opposed to the hand-crafted, custom-made "luxury cars" made in other labs.
"This allows us not to compare different brains but to compare different drivers," Hartung said, referring to different experiments that could be performed on identical brain models.
Hartung said his lab's mini-brains have a variety of glia cells (which support neurons) such as astrocytes and Schwann cells, as well as oligodendrocytes, which form the insulating myelin sheaths that enable nerve impulses — all in proportions similar to those found in the human brain.
The mini-brains' three-dimensional structure and ability to carry neurotransmitters — chemical messengers such as dopamine that enable communication between neurons — provide a simple but relatively realistic platform to study what goes wrong in the brain in, say, drug addiction and how the problem can be remedied.
Hartung said his group accomplishes this by starting with a type of adult skin cell called a fibroblast, inducing those cells back to the state of neural stem cells that give rise to all the cells of the brain and nervous system, and then growing them in a gently rolling, vibrating environment to create the 3D-ball structure. The lab has grown thousands of these mini-brains, each with about 20,000 cells.
Missing for now in the mini-brain but present in a real brain, Hartung said, are immune cells, which come from a different line of stem cells. He said he hopes to incorporate these types of cells soon. Hartung said he may have a working mini-brain for laboratory experimentation by the end of 2016, which could be mailed to any laboratory in the world. [Top 3 Techniques for Creating Organs in the Lab]
Once the mini-brain model is mature, "no one should have the excuse to still use animal models, which come with tremendous disadvantages for brain studies in particular," Hartung said. "While rodent models have been useful, we are not 150-lb. rats. And even though we are not balls of cells, either, you can often get much better information from these balls of cells than from rodents."
Hartung added that upward of 95 percent of therapeutic drugs for neurological orders that look promising in rodent studies fail in humans because of the intrinsic brain differences between the species.
The mini-brain model is well-suited for studying brain addiction, in that scientists can study how drugs can destroy glia cells. Such destruction leads to the death of neurons and poorer transmission of neural impulses, Hartung said.
Hartung's group is investigating the possibility of using the mini-brain to study the effect of Zika virus on a developing brain.
Follow Christopher Wanjek @wanjek for daily tweets on health and science with a humorous edge. Wanjek is the author of "Food at Work" and "Bad Medicine." His column, Bad Medicine, appears regularly on Live Science.

Christopher Wanjek is a Live Science contributor and a health and science writer. He is the author of three science books: Spacefarers (2020), Food at Work (2005) and Bad Medicine (2003). His "Food at Work" book and project, concerning workers' health, safety and productivity, was commissioned by the U.N.'s International Labor Organization. For Live Science, Christopher covers public health, nutrition and biology, and he has written extensively for The Washington Post and Sky & Telescope among others, as well as for the NASA Goddard Space Flight Center, where he was a senior writer. Christopher holds a Master of Health degree from Harvard School of Public Health and a degree in journalism from Temple University.
Most Popular


