Bizarre Properties of Glass Revealed
Scientists have made a breakthrough discovery in the bizarre properties of glass, which behaves at times like both a solid and a liquid.
The finding could lead to aircraft that look like Wonder Woman's plane. Such planes could have wings of glass or something called metallic glass, rather than being totally invisible.
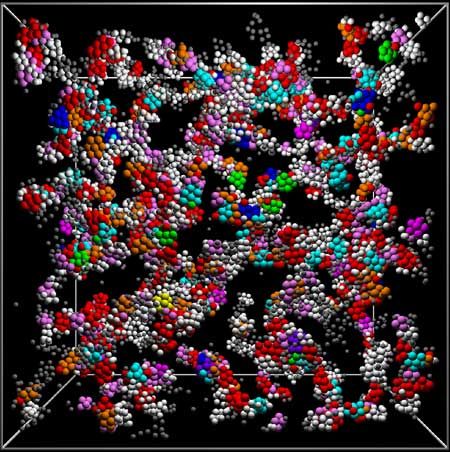
The breakthrough involved solving the decades-old problem of just what glass is. It has been known that that despite its solid appearance, glass and gels are actually in a "jammed" state of matter — somewhere between liquid and solid — that moves very slowly. Like cars in a traffic jam, atoms in a glass are in something like suspended animation, unable to reach their destination because the route is blocked by their neighbors. So even though glass is a hard substance, it never quite becomes a proper solid, according to chemists and materials scientists.
Work so far has concentrated on trying to understand the traffic jam, but now Paddy Royall from the University of Bristol, with colleagues in Canberra and Tokyo, has shown that glass fails to be a solid due to the special atomic structures that form in a glass when it cools.
Icosahedron jams
Some materials crystallize as they cool, arranging their atoms into a highly regular pattern called a lattice, Royall said, but although glass "wants" to be a crystal, as it cools the atoms become jammed in a nearly random arrangement, preventing it from forming a regular lattice.
In the 1950s, Sir Charles Frank in the Physics Department at Bristol suggested that the arrangement of the "jam" should form what is known as an icosahedron, but at the time he was unable to prove it.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
An icosahedron is like a 3-D pentagon, and just as you cannot tile a floor with pentagons, you cannot fill 3-D space with icosahedrons, Royall explained. That is, you can't make a lattice out of pentagons.
When it comes to glass, Frank thought, there is a competition between crystal formation and pentagons that prevents the construction of a crystal. If you cool a liquid down and it makes a lot of pentagons and the pentagons survive, the crystal cannot form.
It turns out that Frank was right, Royall said, and his team proved this experimentally. You can't watch what happens to atoms as they cool because they are too small, so Royall and his colleagues used special particles called colloids that mimic atoms, but are large enough to be visible using state-of-the-art microscopy. The team cooled some down and watched what happened.
What they found was that the gel these particles formed also "wants" to be a crystal, but it fails to become one due to the formation of icosahedra-like structures — exactly as Frank had predicted.
"It is the formation of these structures that underlie jammed materials and explains why a glass is a glass and not a liquid — or a solid," Royall said.
The findings are detailed in the June 22 issue of the journal Nature Materials. The research was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology as well as the Royal Society.
Preventing jetliner disasters
Knowing the structure formed by atoms as a glass cools represents a major breakthrough in the understanding of meta-stable materials and will allow further development of new strong yet light materials called metallic glasses, he said, already used to make some golf clubs. This stuff is generally shiny black in color, not transparent, due to having a lot of free electrons (think of mercury in an old thermometer).
Metals normally crystallize when they cool, however stress builds up along the boundaries between crystals, which can lead to metal failure.
For example, the world's first jetliner, the British built De Havilland Comet, fell out of the sky due to metal failure. When metals are be made to cool with the same internal structure as a glass and without crystal grain boundaries, they are less likely to fail, Royall said. Metallic glasses could be suitable for a whole range of products, beyond golf clubs, that need to be flexible such as aircraft wings and engine parts, he said.
Glass is not what it seems
Royall is part of a group of scientists who think that if you wait long enough, perhaps billions of years, all glass will eventually crystallize into a true solid. In other words, glass is not in an equilibrium state, (although it appears that way to us during our limited lifetimes). "This is not universally accepted," Royall told LiveScience. "Our work will go some way to making that point more accepted. I think there is a growing weight of evidence that certainly many glasses 'want' to be a crystal." Still, glass "looks like a liquid and this is one of the great riddles that we have gone some way to solving," Royall said. "It has always been thought that glass has same structure as a liquid, and that's why it looks like it. It does not have same structure as liquid."
- Video: Roboswift, the Flying Bird
- 10 Technologies That Will Change Your Life
- Great Inventions: Quiz Yourself
Robin Lloyd was a senior editor at Space.com and Live Science from 2007 to 2009. She holds a B.A. degree in sociology from Smith College and a Ph.D. and M.A. degree in sociology from the University of California at Santa Barbara. She is currently a freelance science writer based in New York City and a contributing editor at Scientific American, as well as an adjunct professor at New York University's Science, Health and Environmental Reporting Program.