Facts About Nitrogen

Nitrogen is essential to life on Earth. It is a component of all proteins, and it can be found in all living systems. Nitrogen compounds are present in organic materials, foods, fertilizers, explosives and poisons. Nitrogen is crucial to life, but in excess it can also be harmful to the environment.
Named after the Greek word nitron, for "native soda," and genes for "forming," nitrogen is the fifth most abundant element in the universe. Nitrogen gas constitutes 78 percent of Earth's air, according to the Los Alamos National Laboratory. On the other hand, the atmosphere of Mars is only 2.6 percent nitrogen.
In its gas form, nitrogen is colorless, odorless and generally considered as inert. In its liquid form, nitrogen is also colorless and odorless, and looks similar to water, according to Los Alamos.
Just the facts
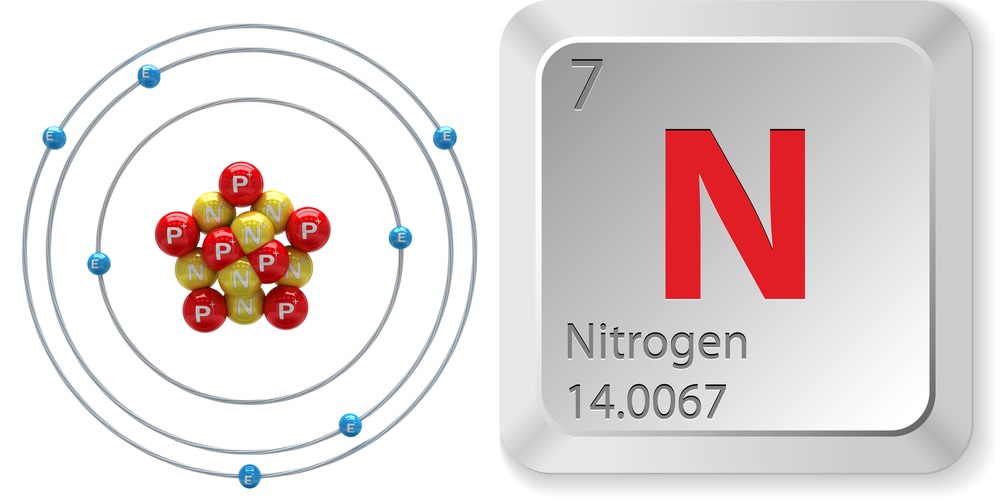
- Atomic number (number of protons in the nucleus): 7
- Atomic symbol (on the Periodic Table of Elements): N
- Atomic weight (average mass of the atom): 14.0067
- Density: 0.0012506 grams per cubic centimeter
- Phase at room temperature: Gas
- Melting point: minus 321 degrees Fahrenheit (minus 210 degrees Celsius)
- Boiling point: minus 320.42 F (minus 195.79 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 16 including 2 stable ones
- Most common isotopes: Nitrogen-14 (Abundance: 99.63 percent)
Fertilizer component
Nitrogen was discovered in 1772 by chemist and physician Daniel Rutherford, when he removed oxygen and carbon dioxide from air, demonstrating that the residual gas would not support living organisms or combustion, according to the Los Alamos National Laboratory. Other scientists, including Carl Wilhelm Scheele and Joseph Priestly, were working on the same problem, and called nitrogen "burnt" air, or air without oxygen. In 1786, Antoine Laurent de Lavoisier, called nitrogen "azote," which means "lifeless." This was based on the observation that it is the part of air cannot support life on its own.
One of the most important nitrogen compounds is ammonia (NH3), which can be produced in the so-called Haber-Bosch process, in which nitrogen is reacted with hydrogen. The colorless ammonia gas with a pungent smell can be easily liquefied into a nitrogen fertilizer. In fact, about 80 percent of ammonia that is produced is used as fertilizer. It is also used as a refrigerant gas; in the manufacture of plastics, textiles, pesticides and dyes; and in cleaning solutions, according to the New York Department of State.
The nitrogen cycle
The nitrogen cycle, in which atmospheric nitrogen is converted into different organic compounds, is one the most crucial natural processes to sustain living organisms. During the cycle, bacteria in the soil process or "fix" atmospheric nitrogen into ammonia, which plants need in order to grow. Other bacteria convert the ammonia into amino acids and proteins. Then animals eat the plants and consume the protein. Nitrogen compounds return to the soil through animal waste. Bacteria convert the waste nitrogen back to nitrogen gas, which returns to the atmosphere.
In an effort to make crops grow faster, people use nitrogen in fertilizers. However, the excessive use of those fertilizers in agriculture has had devastating consequences for the environment and human health, as it has contributed to the pollution of groundwater and surface waters. According to the U.S. Environmental Protection Agency (EPA), nutrient pollution caused by excess nitrogen and phosphorus in the air and water, is one of the most widespread, costly and challenging environmental problems.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Tackling the imbalance
One of the solutions to the issue of excessive nitrogen lies in sustainable agriculture, organic farming and raising the awareness of these environmental issues among farmers, according to Randy A. Dahlgren, a professor of soil science at University of California, Davis.
"The idea would be to try to eliminate the use of these commercial fertilizers and instead use organic waste," for instance, animal waste, he said. Another step would be to use slow-release fertilizers that have plastic coatings on them and, instead of releasing the nitrogen right away, the release of nitrogen occurs gradually throughout the growing season, "trying to match the nitrogen release from the plastic coated fertilizer with the needs of the plant," he said.
Microbiologists at the University of Alberta in Canada and the University of Vienna in Austria may have discovered another solution. In August 2017, the researchers announced they had identified an ammonia-oxidizing microbe called Nitrospira inopinata.
According to the researchers, Nitrospira inopinata is an ammonium sponge, essentially outperforming nearly every other type of bacteria and archaea (single-celled organisms) in the oxidation of ammonium in the environment. Because this microbe is such an efficient oxidizer, it may produce less nitrous oxide in the process.
The new findings, which appear in the scientific journal Nature, could have important implications for climate change research. The researchers are ready to put this microbe to the test through a variety of practical applications that could reduce ammonium levels in the soil, water and atmosphere. Some of these applications could involve changes to our drinking water, wastewater treatments and soil purification, according to the University of Alberta.
Who knew?
- Even though the term "nitrogen" is used in English to refer to the element, Lavoisier's term "azote" is still used in French, and its form is present in "azoto" in Italian or "azot" in Polish.
- Liquid nitrogen is frequently used as a refrigerant, for instance, to store sperm, eggs and other cells used in medical research or fertility clinics, according to the Royal Society of Chemistry.
- Liquid nitrogen is also used to quickly freeze foods and help preserve their flavor, texture, moisture and flavor.
- Nitrogen constitutes 95 percent of the atmosphere of Titan (the largest moon of Saturn), according to the Jet Propulsion Laboratory.
- Nitrogen gas plays a role in the formation of an aurora — a natural display of light in the sky that can be predominantly observed Arctic and Antarctic regions — which occurs when fast-moving electrons from space collide with oxygen and nitrogen in our atmosphere, according to NASA.
- Nitrogen gas can be obtained by heating a water solution of ammonium nitrate (NH4NO3), a crystalline solid that is commonly used in fertilizer.
- About 150 tons of ammonia are produced every year using the Haber process, according to Royal Society of Chemistry.
- Nitrogen in the form of ammonium chloride, NH4Cl, was produced in ancient Egypt by heating a mixture of animal excrement, urine and salt, according to Royal Society of Chemistry.
- Nitroglycerin, a violent explosive used in the production of dynamite, is an oily, colorless liquid that contains nitrogen, oxygen and carbon.
Additional reporting by Traci Pedersen, Live Science contributor.
Additional resources
- This website describes what happens when you try to put different objects into liquid nitrogen.
- This infographic illustrates nitrogen pollution in Chesapeake Bay.