Breakthrough: Eye Cells Regenerated in Live Mice
Scientists have for the first time regrown retina cells in live mammals. The mouse study offers hope for similar success in human eye cells.
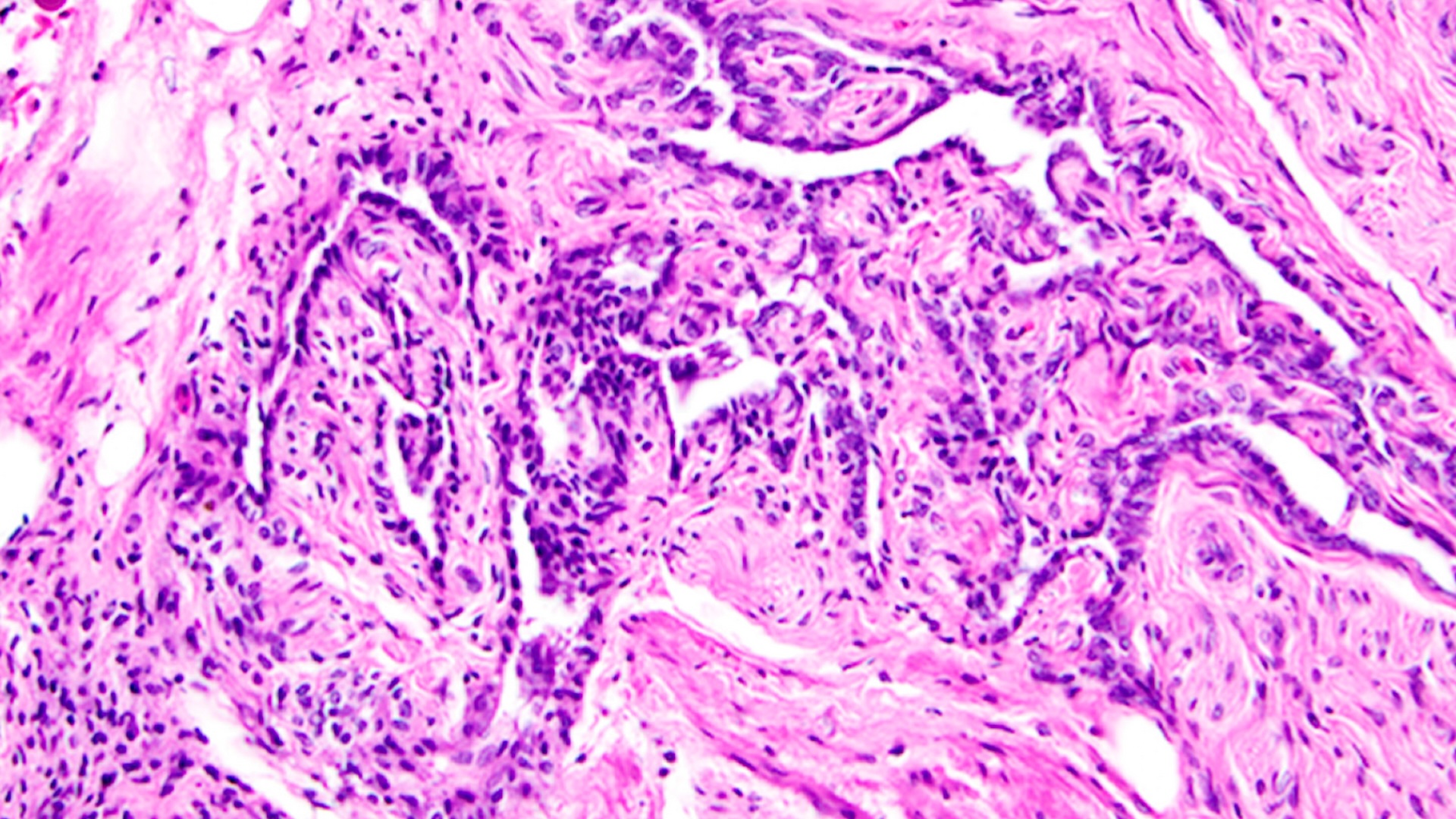
Located in the back of the eye, the retina's role in vision is to convert light into nerve impulses to the brain. Previous work had shown that retina nerve cells called Müller glia cells could be grown in a lab dish.
"This type of cell exists in all the retinas of all vertebrates," said Tom Reh of the University of Washington, "so the cellular source for regeneration is present in the human retina."
Reh said further study could lead to new treatments for human vision loss from retina-damaging diseases, like macular degeneration.
The research will be detailed this week in the Early Edition of the Proceedings of the National Academy of Sciences.
Birds, which are warm-blooded like mammals, have some limited ability to regenerate retinal nerve cells. But fish, which are cold-blooded, can generate all types of retinal nerve cells, Reh said.
Getting the cells to regrow in mice — considered a good model for human biology — was not easy, however.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The researchers injected a substance into the retina to eliminate ganglion cells (a type of nerve cell found near the surface of the retina) and amacrine cells. Then by injecting the eye with some chemicals, including growth factors and insulin, they were able to stimulate the Müller glia cells to restart their dividing engines and begin to proliferate across the retina.
Many of the progenitor cells arising from the dividing Müller glia cells, the researchers observed, died within the first week after their production. However, those that managed to turn into amacrine cells survived for at least 30 days.
"It's not clear why this occurs," the researchers wrote, "but some speculate that nerve cells have to make stable connections with other cells to survive."