The Enduring Mystery of Light

It goes through walls, but slows to a standstill in ultra-cold gases. It carries electronic information for radios and TVs, but destroys genetic information in cells. It bends around buildings and squeezes through pinholes, but ricochets off tiny electrons.
It's light. And although we know it primarily as the opposite of darkness, most of light is not visible to our eyes. From low energy radio waves to high energy gamma rays, light zips around us, bounces off us, and sometimes goes through us.
Because it is so many things, defining light is a bit of a philosophical quandary. It doesn't help that light continue to surprise us, with novel materials that alter light's speed and trajectory in unexpected ways.
Is it a wave?
What ties together microwaves, X-rays and the colors of the rainbow is that they are all waves — electromagnetic waves to be exact. The substance that sloshes back and forth isn't water or air, but a combination of electric and magnetic fields.
These fluctuating fields exert forces on charged particles — sometimes causing them to bob up and down like buoys in the ocean.
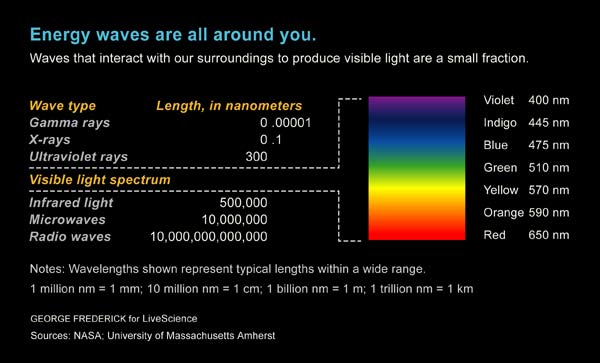
What separates all the various forms of light is wavelength. Our eyes are sensitive to light with wavelengths between 750 nanometers (red) and 380 nanometers (violet), where a nanometer is one billionth of a meter, or about the size of a single molecule.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
But the visible spectrum — seen through a prism — is only a small chunk of the entire electromagnetic spectrum. The wavelength of light ranges from hundreds of miles for long radio waves to one millionth of a nanometer for gamma rays.
The energy of light is inversely proportional to the wavelength, such that gamma rays are a billion billion times more energetic than radio waves.
Or is it a particle?
But waves are not the whole story. Light is composed of particles called photons. This is most obvious with higher energy light, like X-rays and gamma rays, but it is true all the way down to radio waves.
The classic example of particleness is the photoelectric effect, in which light hitting a metal sheet causes electrons to fly out of the surface. Surprisingly, light longer than a certain wavelength cannot liberate electrons, no matter how bright the source is.
A strict wave theory of light cannot explain this wavelength threshold, since many long waves should pack the same total energy as a few short waves.
Albert Einstein deciphered the mystery in 1905 by assuming that particles of light smacked into the electrons, like colliding billiard balls. Only particles from short wavelength light can give a hard enough kick.
Despite this success, the particle theory never replaced the wave theory, since only waves can describe how light interferes with itself when it passes through two slits. We therefore have to live with light being both a particle and a wave — sometimes acting as hard as a rock, sometimes as soft as a ripple.
Physicists rectify light's split personality by thinking in terms of wave packets, which one can imagine as a group of light waves traveling together in a tight, particle-like bundle.
Making a spectacle
Instead of worrying about what light is, it might be better to concentrate on what light does. Light shakes, twists and shoves the charged particles (like electrons) that reside in all materials.
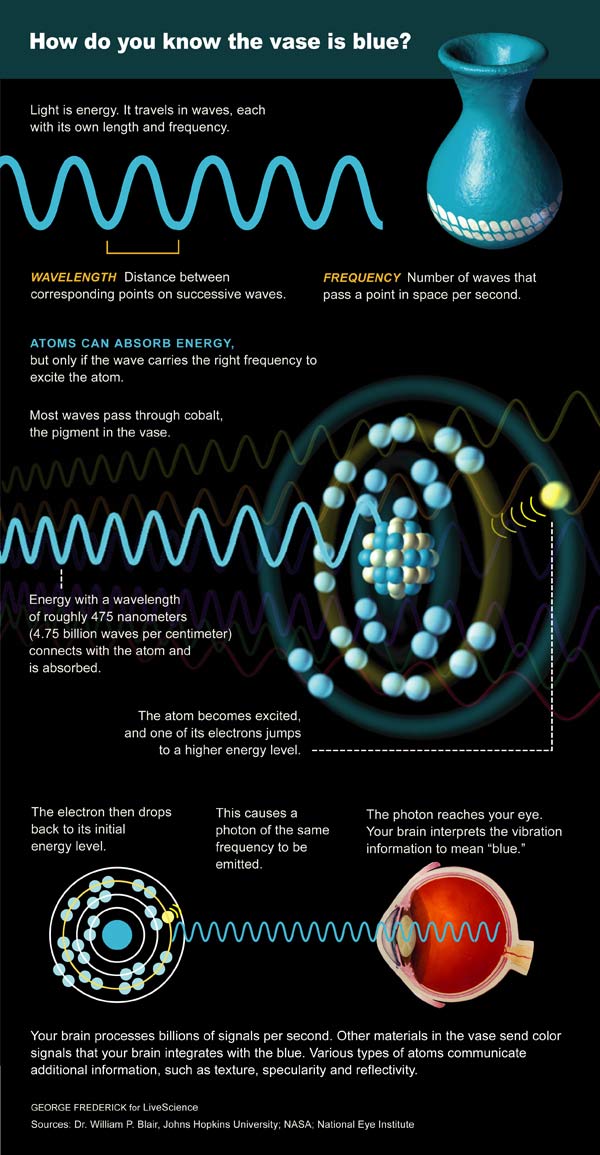
These light actions are wavelength-specific. Or to say it another way, each material responds only to a particular set of wavelengths.
Take an apple, for instance. Radio waves and X-rays go essentially straight through it, whereas visible light is stopped by various apple molecules that either absorb the light as heat or reflect it back out.
If the reflected light enters our eyes, it will stimulate color receptors (cones) that are specifically "tuned" to either long, medium or short wavelengths. The brain compares the different cone responses to determine that the apple reflects "red" light.
Here are some other examples of light's specific activities.
- Radio waves from a local station cause the free electrons in a radio's antenna to oscillate. Electronics tuned to the station's frequency (or wavelength) can decode the oscillating signal into music or words.
- A microwave oven heats food from the inside out because microwaves penetrate the surface to rotate water molecules contained in the food. This molecular shuffling generates heat.
- Standing next to a camp fire, infrared light vibrates molecules in our skin to make us warm. Conversely, we constantly lose heat when these same molecules emit infrared light.
- In sunlight, several visible and ultraviolet wavelengths are missing, or dark. These "shadows" are due to the capture of photons by atoms, like hydrogen and helium, that make up the sun. The captured photon energy is used to boost the atoms' electrons from one energy level to another.
- An X-ray image of a skeleton is due to the fact that X-rays pass through soft tissue but are blocked by dense bone. However, even when just passing through, X-rays and gamma-rays ionize molecules along their path, meaning they strip electrons from the molecules. The ionized molecules can directly or indirectly damage DNA in a cell. Some of these genetic alterations may lead to cancer.
All this shows that light wears many different hats in its manipulation of matter. It is perhaps fitting then that light's true identity — wave or particle — is unanswerable.











