Atom Breaks Rules, Beats Friction
Scientists have found a molecule that can spin freely in liquid, clearing out water like a person swinging suitcases would clear a crowded room.
The molecule spins without causing friction [Video]. That shouldn't be possible, according to a chemical physics theory. The finding could alter the way scientists think about chemical reactions in liquids.
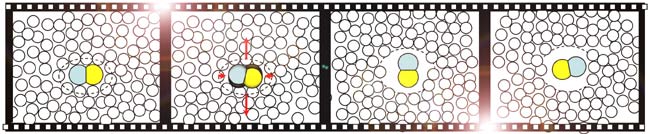
Researchers hit a drop of iodine cyanide and water with pulses from an ultraviolet laser, exciting one type of molecule to reconfigure into a small, peanut shape with a carbon atom on one end, a nitrogen atom on the other.
The molecule heated up to 8,000 degrees Fahrenheit (4,427 Celsius) and started spinning at a furious 270 trillion rotations per minute.
Outta my way
Within the first quarter-turn, the molecule created a shock wave that kicked away surrounding water molecules. The peanut molecule created a nearly frictionless zone for itself in the 10-trillionths of a second the reaction lasted.
"If you give it enough spin, it pushes all the guys around it away, and it builds itself a little bubble," said study coauthor Stephen Bradforth of the University of Southern California. "It's destroyed the friction in the liquid around it by completely reshaping its environment."
Get the world’s most fascinating discoveries delivered straight to your inbox.
After the molecule completed about 10 rotations, the shock dwindled and the water molecules rushed back in.
Despite its fleeting nature, the reaction managed to smash the linear response theory, a chemistry model that states such a thing can't happen in a liquid environment.
"You can see molecules behave this way in gases, but not in liquids," said study coauthor Richard Stratt, a chemical theorist at Brown University.
Breaking other laws
The molecule's activity also runs against Newton's third law of motion, which states that for every action there is an equal, but opposite, reaction. In the new experiment, there water molecules are displaced, but they don't in turn do anything to the peanut molecule.
Friction is important in chemistry. Molecules rub, grind, and bang against each other they generate heat that speeds up reactions. Friction in gas reactions is reduced due to the relatively far distances between molecules, but the close proximity of molecules in liquid form makes friction nearly unavoidable.
Although the discovery has no immediate practical use, it changes the way scientists think about the 90 percent of all chemical reactions that take place in liquid, Bradforth said. One potential use could be to manipulate reactions by isolating molecules from their surroundings and reducing the production of useless byproducts.
"The main reason we're so excited by these results is that friction is how energy is shuttled around in chemical reactions," Stratt told LiveScience. "If it doesn't operate or it operates differently than we always thought, that makes us wonder if there are entirely new ways we ought to thinking about how chemical reactions take place."
The research is detailed in the March 31 issue of the journal Science.
- Video: See the Molecule Spin
- How Dolphins Spin, and Why
- Caution: Slippery Nanotubes Ahead
- Spinning into Control?
 Live Science Plus
Live Science Plus





