New Tanks Could Make Natural Gas Practical for Cars
In just a few years, it may be more convenient for cars and SUVs to run on natural gas, thanks to a recently developed storage technology.
Natural gas, or methane, has been touted as a viable alternative fuel for vehicles. While it is still a fossil fuel, it is cleaner burning than gasoline, and so emits fewer greenhouse gases.
“The amount of carbon dioxide that comes out of the exhaust is really so much less than that from gasoline,” said the storage system’s creator, Peter Pfeifer of the University of Missouri-Columbia.
Currently, natural gas is most practical when fueling large vehicles such as buses and trucks because it must be stored in large tanks that would take up all the storage space in smaller cars.
“It just makes it unattractive for many people” to use in their cars, Pfeifer said.
From corncob to charcoal
Methane molecules generally like their personal space and are difficult to pack closely together, so natural gas must be subjected to high pressures to store enough of it to fuel a vehicle over long enough distances.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
To withstand these high pressures, natural gas tanks are big, thick-walled and cylindrical, so they would take up the entire trunk of an average sedan.
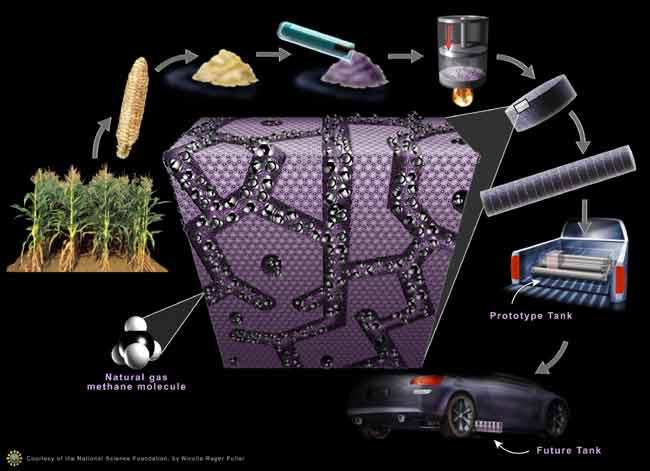
The goal of Pfeiffer’s research was to develop a way to hold the natural gas at lower pressures, which would shrink tank sizes. To do this, Pfeiffer developed a way to transform corncob waste into carbon briquettes that act like a sponge to suck up and store natural gas at higher densities.
Tiny pores in the corncobs store the natural gas “in a way which makes the methane molecules very happy to be close to each other,” said Pfeifer.
To transform the corncobs into briquettes, they are heated in the absence of air, essentially turning them into charcoal. Meanwhile, a chemical process “drills” extremely tiny holes and tunnels in the briquettes that can exert strong forces to hold the methane in place.
Why corncob?
Pfeifer and his team chose to use corncob because it is an abundant waste product in the Midwest.
“Our goal was basically to do it from something that we could supply the whole nation with, without having to import anything,” Pfeifer told LiveScience.
Other groups have tried to transform coconuts, olive pits or other plant material readily available near them into carbon briquettes, but have not reached the storage capacity Pfeifer’s system did.
His team’s tank operates at one-seventh the pressure of a normal gas tank; it is smaller than conventional high-pressure natural gas tanks and has a flat, rectangular shape.
“It’s something that easily, without you ever noticing it, would fit under the floor of your car or could be put into some other unused space of your vehicle,” said Pfeifer.
The physicists tested their tank out on one of the fleet of trucks that Kansas City had already outfitted with compressed natural gas tanks.
“It’s just been performing very well,” said Pfeifer.
Future challenges
In addition to being a cleaner alternative to gasoline, natural gas is also currently cheaper, but some experts say the price could go up as people use it more. Natural gas also does not provide a complete solution to the problem of finding alternative fuels.
“It is a limited resource, it’s not renewable,” said Michael Bobker of the City University of New York's Institute for Urban Systems, who was not affiliated with the project.
But Pfeifer remains optimistic about the prospects of his technology, once it is further refined.
“With natural gas, I think with a concerted effort on the part of industry and maybe a national agenda, you know, coming from Washington or what not, I think these things could be on the road in less than five years,” he said.

Andrea Thompson is an associate editor at Scientific American, where she covers sustainability, energy and the environment. Prior to that, she was a senior writer covering climate science at Climate Central and a reporter and editor at Live Science, where she primarily covered Earth science and the environment. She holds a graduate degree in science health and environmental reporting from New York University, as well as a bachelor of science and and masters of science in atmospheric chemistry from the Georgia Institute of Technology.
Crop circles surround Iraq's multicolored 'Sea of Salt' after years of drought — Earth from space
Watch humanlike robot with bionic muscles dangle as it twitches, shrugs and clenches its fists in creepy video
'The parasite was in the driver's seat': The zombie ants that die gruesome deaths fit for a horror movie










