New Human Bone Made of Seaweed and Crustaceans
Seaweed, crustacean shells, and a patient's own cells may allow doctors to improve bone grafts.
To fill gaps in a bone—which can result from accidents or surgery, especially when some kinds of tumors are cut away— surgeons will often craft a scaffold made of carbon nanotubes or other artificial material. Once in place, cells from the surrounding bone find their way to the scaffold and reproduce, forming new bone.
But there's a catch, or more accurately, two, says Hockin Xu, a research scientist at the National Institute of Standards and Technology.
"New bones only form on the surface layer of the implants," he explains. "There is no new bone in the middle of the implant. As a result the middle of the implant is very weak." Another problem: Scaffolds constructed outside the body and then dropped into place never fit perfectly.
A better way
Now Xu and his colleagues have developed a better way to bridge bone breaches. In this system bone cells grow from inside the scaffold, producing a structure that is more consistently solid and that eventually morphs into natural bone.
This system combines a cement that is made of calcium phosphate, a mineral found in bone, along with a commercial mesh that gradually dissolves in the body. Surgeons can either form the cement or inject it straight into the gap. The biodegradable mesh reinforces the cement so that it is strong enough to survive until natural-bone reinforcements arrive.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Adding chitosan, a biopolymer that is extracted from crustacean shells, makes the structure even stronger.
So that bone cells aren't excluded from the scaffold's inner reaches, cells are mixed right into the cement. And so the body doesn't reject these cells, the patient's own bone cells are added. These cells are cultured in a laboratory from samples drawn from the patient. Culturing enough cells takes a week or two.
Mixing and setting
But the whole process of mixing the cement and the period that it is settingwaiting for it to setis damaging forcan damage the live cells in the mix any bone cells, ß confusing whether or not they come from the patient.
"If we mix the cells with the cement, the cells all die," Xu said. To separate the fragile cells from the harsh cement, the scientists make a sort of a medical M&M in which bone cells are the center and a natural polymer that is squeezed out of seaweed is the coating.
"Once the cement is set and it is safe for the cells, the beads then dissolve away," he explained.
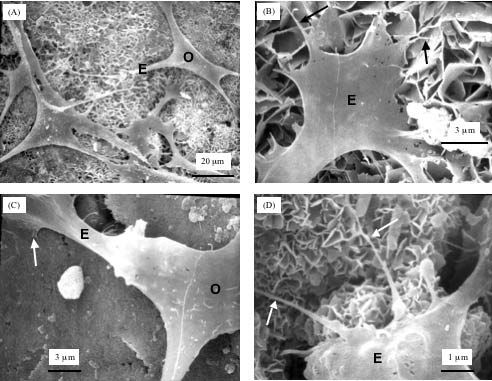
Not only do the beads protect the bone cells, when they dissolve they leave one-millimeter "macropores"—the relatively large spaces that represent the empty parts of the scaffold—that the bone cells grow in. As the cells gradually grow, Xu said, all of the components of the scaffold, the mesh, cement, and beads, will dissolve and be replaced by bone.
"New bone is quite strong," he said. "So as the macroporous implant is filled with new bone, it becomes stronger over time."
The technology is still in early stages of development. Among the refinements that Xu's team hopes to make is to increase the rate at which the beads dissolve and to reduce the beads' size, which would make the spaces in the scaffolds smaller and so the structures stronger.