Molecular Clues Hint at What Really Caused the Black Death
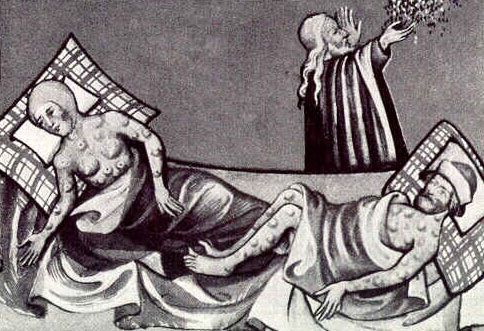
The Black Death arrived in London in the fall of 1348, and although the worst passed in less than a year, the disease took a catastrophic toll. An emergency cemetery in East Smithfield received more than 200 bodies a day between the following February and April, in addition to bodies buried in other graveyards, according to a report from the time.
The disease that killed Londoners buried in East Smithfield and at least one of three Europeans within a few years time is commonly believed to be bubonic plague, a bacterial infection marked by painful, feverish, swollen lymph nodes, called buboes. Plague is still with us in many parts of the world, although now antibiotics can halt its course. [Pictures of A Killer: A Plague Gallery]
But did this disease really cause the Black Death? The story behind this near-apocalypse in 14th century Europe is not clear-cut, since what we know about modern plague in many ways does not match with what we know about the Black Death. And if plague isn't responsible for the Black Death, scientists wonder what could've caused the sweeping massacre and whether that killer is still lurking somewhere.
Now, a new study using bone and teeth taken from East Smithfield adds to mounting evidence exhumed from Black Death graves and tantalizes skeptics with hints at the true nature of the disease that wiped out more than a third of Europeans 650 years ago.
This team of researchers approached the topic with open minds when they began looking for genetic evidence of the killer.
"Essentially by looking at the literature on the Black Death there were several candidates for what could have been the cause," said Sharon DeWitte, one of the researchers who is now an assistant professor of anthropology at the University of South Carolina.
Their first suspect: Yersinia pestis, the bacterium that causes modern plague, including bubonic plague.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The speed of plague
In 1894, Alexander Yersin and another scientist separately identified Y. pestis during an epidemic in Hong Kong. Years later the bacterium was given his name. Yersin also connected his discovery to the pestilence that swept Europe during the Black Death, an association that has stuck.
One problem, however, is that compared to the wildfire-like spread of the Black Death, the modern plague moves more leisurely. The modern plague pandemic began in the Yunnan Province of China in the mid-19th century, then spread to Hong Kong and then via ship, to India, where it exacted the heaviest toll, and to San Francisco in 1899, among many other places.
The disease that caused the Black Death is believed to have traveled much quicker, arriving in Europe from Asia in 1347, after the Golden Horde, a Mongol Army, catapulted plague-infected bodies into a Genoese settlement near the Black Sea. The disease traveled with the Italian traders and later appeared in Sicily, according to Samuel Cohn, a professor of medieval history at the University of Glasglow and author of "The Black Death Transformed: Disease and Culture in the Early Renaissance Europe" (Bloomsbury USA, 2003).
By about 1352, roughly five years after arriving in Europe, it had not only spread across the continent, the worst of the disease had already run its course.
This wave of devastation becomes particularly surprising considering the complicated and time-consuming process by which plague has been thought to spread. You can't catch bubonic plague from another person; instead, the process involves two classic villains: rats and fleas.
Once a flea bites a rat infected with plague, the pathogen Y. pestis grows in its gut. After about two weeks, the bacteria block the valve that opens into the flea's stomach. The starving flea then bites its host, by now probably a new, healthy rat or a person, more aggressively in an attempt to feed. All the while, the flea tries to clear out the bacterial obstruction and so regurgitates the pathogen onto the bite wounds, according to Ken Gage, chief of flea-borne disease activity with the U.S. Centers for Disease Control and Prevention.
The bulk of cases during the modern plague pandemic are believed to have been spread by rats and their fleas, according to Gage. The last rat-borne plague epidemic in the U.S. occurred in 1925; wild rodents have since become the primary source for infections. However, rat-associated outbreaks continue to occur in developing countries, according to the CDC.
Fast, furious and unfamiliar
Not only has the disease slowed down, it also seems to have become more restrained. The Black Death wiped out at least 30 percent of Europe's population at the time. But the peak of the modern pandemic, in India, killed less than 2 percent of the population, DeWitte has calculated from census data.
The list of discrepancies goes on: There is evidence the Black Death spread directly between humans — no rats and their fleas involved — and to areas where rats and their fleas didn't even live. In fact, archaeological and documentary evidence indicates rats were scarce during the mid-14th century.
What's more, bubonic plague doubters point out, deaths during the Black Death appear to have followed a different seasonal cycle than plague deaths in modern times. Some also point to discrepancies in the symptoms.
Alternative theories
With the plague's role called into question, other theories have been offered to fill the gap.
"There is a lot of evidence that suggests that Yersinia pestis may not have been the causative agent for the Black Death, and it was likely something else, and something else that is out there right now," said Brian Bossak, an environmental health scientist at Georgia Southern University.
He is among those who suspect a hemorrhagic virus — which causes bleeding and fever, like ebola — swept through 14th-century Europe. The high lethality, rapid transmission and periodic resurgences seen in the Black Death are characteristic of a virus, according to Bossak, who frames this as a question in urgent need of resolution.
"Who knows if it won't happen again," he said. "It seems like every so often some disease comes out of nowhere."
Two other proponents of the virus theory, Susan Scott and Christopher Duncan of the University of Liverpool in the United Kingdom, have pointed to a possible genetic legacy left by a viral Black Death: a mutation, known as CCR5-delta32, found among Europeans, particularly those in the north. This mutation confers resistance against HIV, another virus, but does not prevent plague. It's possible that by passing over those with this mutation, the Black Death selected for this change in the genetic code, making it more common among Europeans, they argue.
To at least some degree, an alternative form of plague, pneumonic plague, offers a solution. While bubonic is the most common form of plague, plague can also infect the lungs, causing high fever, cough, bloody sputum and chills. This infection can spread person-to-person, and without antibiotic treatment it is nearly 100 percent fatal. Outbreaks have occurred in modern times, and it can develop as a result of a bubonic infection. But, it is unclear how much of a role it played in the Black Death — some evidence suggests it is not as contagious as commonly thought.
Rats and fleas
The Black Death just doesn't appear to have behaved the way the typical, modern rat-associated plague does, according to Gage, the flea expert. Even so, he says he is convinced that bubonic plague was responsible.
A group of French researchers found another possible insect carrier for the Black Death: lice. They were able to transmit fatal plague infections from sick rabbits to healthy ones via human body lice that fed on the rabbits. Substitute humans for bunnies, and this scenario offers a simpler, more cold-climate-friendly explanation than the conventional rat-flea model.
But fleas aren't out of the picture yet. Gage and his colleagues have found that many species of flea — including the Oriental rat flea, a widespread and important spreader of plague — can begin transmitting the infection much sooner than thought, before the bacterium blocks off its stomach. This supports the idea that a species of human-inhabiting fleas, whose guts the bacterium can't block well, could have spread the infection from person to person in areas without rats, Gage said. [10 Deadly Diseases That Hopped Across Species]
Plague isn't picky about its warm-blooded victims; it can infect almost any mammal, although some, like humans, cats and rats, become severely ill when infected, according to Gage. The lack of records of massive rat die-offs during the Black Death also calls into question the role rats may have played then.
CSI: Black Death
Plague kills quickly and does not leave marks on the remains that archeologists are digging up centuries later. But in recent years scientists have begun searching for the molecular clues in the remains of the dead, including DNA left by the killer bacterium.
While a number of studies have turned up positive results from graves believed to hold European plague victims, the results haven't always been clear-cut. For instance, a 2004 study of remains in five burial sites, including East Smithfield, was unable to find any evidence of the bacteria.
Looking for evidence of the genetic traces of a pathogen within 650-year-old bones is a challenging proposition, according to Hendrik Poinar, an evolutionary geneticist at McMaster University who worked with DeWitte, then at the University of Albany, on the most recent study. After so many years in the ground, the DNA is damaged and present only in tiny fragments, and, what's more, each sample contains only a miniscule amount of the pathogen — the rest belongs to the person and interlopers like soil bacteria, fungi, insects, even animals.
"You have to come up with a way to pull out the things of interest," Poinar said. So, after screening to detect the presence of Yersinia pestis in the 109 samples from the cemetery in East Smithfield, his lab employed a sort of sensitive fishing technique, using tiny segments of DNA that matched up with segments from a ring of DNA, called a plasmid, found in the bacterium.
Once they had retrieved this DNA, they assembled the full plasmid and compared it with modern versions of the bug. They found this plasmid matched many of the modern versions. They also sequenced a short section of DNA from the bacterium's nucleus and revealed three small changes unseen in the modern strains.
The results prove that a variant of Yersinia pestis infected the victims of the Black Death, the authors write in a recent issue of the journal Proceedings of the National Academy of Sciences.
Same bug, different disease?
This finding comes about a year after another genetic study, led by Stephanie Haensch of Johannes Gutenberg University in Germany, found evidence of two previously unknown strains of Yersinia pestis in the remains of European victims, and hints at a solution that could allow both sides to be right.
"People have always assumed the two diseases were the same," said Cohn, the medieval historian, referring to modern plague and the Black Death. "Even if it is the same pathogen, the diseases are very different."
Bossak, who has questioned the role of plague in the Black Death, agrees.
"This new (study) seems to support these earlier claims, and reinforces the notion that what we know of the epidemiology in modern Y. pestis plague may not fit the Black Death, perhaps because these ancient strains of Y. pestis are no longer present (assuming Y. pestis was indeed the causative agent)," he wrote in an email.
However, Poinar is more cautious. Although they had hoped to find changes that explained why the pathogen might have become less aggressive over the centuries, none have turned up so far. In fact, it's too early to say the changes detected represent any significant difference between the modern and ancient versions of the bacterium, according to him.
"We need the entire genome to say anything about this," Poinar wrote in an email, "and that is for future work."
You can follow LiveScience writer Wynne Parry on Twitter @Wynne_Parry. Follow LiveScience for the latest in science news and discoveries on Twitter @livescience and on Facebook.

In a 1st, trial finds vitamin D supplements may slow multiple sclerosis. But questions remain.

Simple blood tests could be the future of cancer diagnosis