Bacterial 'Vampires' Suck Life Out of Other Microbes
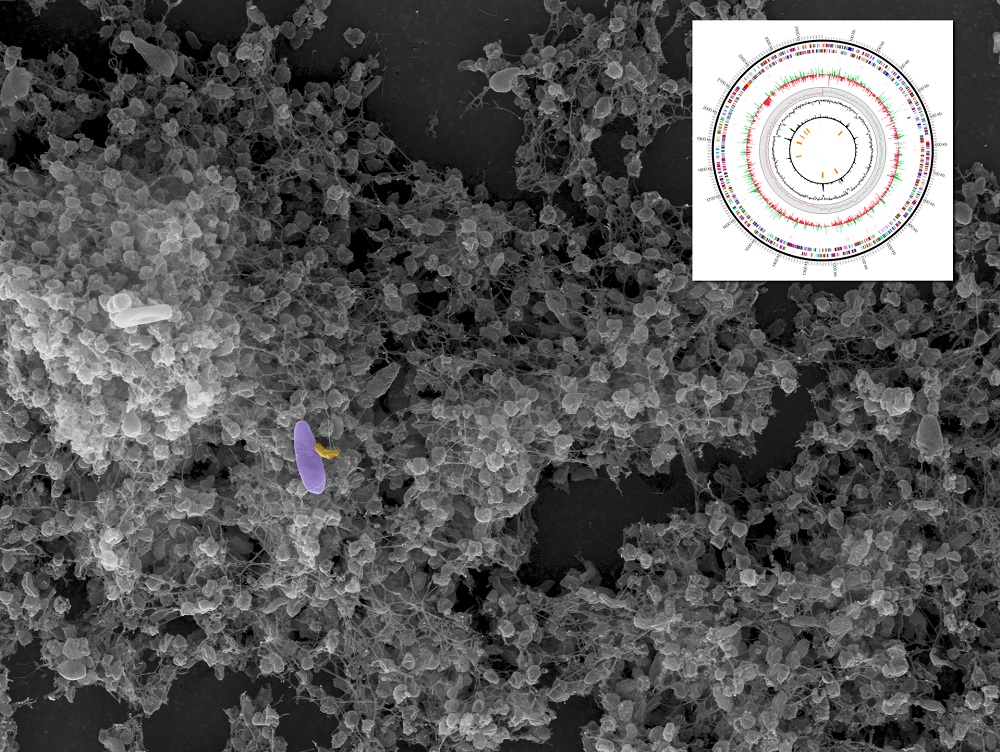
I want to suck your … bacteria? A "vampire" bacteria species, which survives solely by sucking the life out of other bacteria, has had its genome sequenced, revealing its potential to serve as a living antibiotic.
Researchers discovered that the bacterium hunts down prey and attaches itself to the outer layer, or cell wall, of its victim, then sucks them dry of nutrients and energy. In the end, the "victim" bacteria is dead, which could present a very useful strategy for treating bacteria-based human diseases.
"Pathologists may eventually be able to use this bacterium to fight fire with fire, so to speak, as a bacterium that will aggressively hunt for and attack certain other bacteria that are extremely harmful to humans," study researcher Martin Wu said in a statement.
What makes vampire bacteria tick
Micavibrio aeruginosavorus was discovered over 30 years ago in wastewater. It has been difficult to study in the lab, however, because it is contaminated by the other bacteria it feeds on.
In the new study, researchers at the University of Virginia used modern genetic techniques to isolate and sequence the genome of the "vampire bacteria." [The 10 Most Diabolical and Disgusting Parasites]
The genome showed that the vampire bacteria aren't able to survive on their own, even if all vital nutrients are available. That's because they don't have the genes necessary to transport some integral nutrients through their cell wall, so they need to get them directly from other bacteria.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The team also discovered a group of genes that likely play a role in how the vampire bacteria seek out and attach to their prey, and genes that enable them to transport nutrients and energy from the bacteria they eat.
The researchers analyzed what genes were turned on during different phases of the bacteria species' life. When they are "on the hunt," before they settle into their vampirelike existence, genes were turned on that regulated the movement of the bacteria's tail and the sensing of chemical signals in the environment. But during the attach-and-suck-dry phase, the researchers noticed that genes involved in protein production got turned on. Those genes likely helped to build the bridges between the two bacterial cells and for the vampire bacteria to grow and divide in response to the available nutrients.
Vampire treatment
Scientists have known that these kinds of vampire bacteria species attack many different kinds of bacteria, including those that cause chronic lung infections in people with cystic fibrosis. If this vampire could be tamed to kill off these lung infections, it could greatly improve survival of cystic fibrosis patients who often succumb to lung complications of their disease by the time they reach 40, the researchers said.
New approaches like these are needed to control bacterial populations, especially those dangerous to human health, Wu said. Traditional antibiotics breed resistance as the bacteria adapt to the drugs and "escape" their antibacterial effects. This resistance leads to super-bugs, bacteria that are resistant to multiple kinds of drugs.
Using a living antibacterial agent like the vampire bacteria would enable this bacterial "treatment" to adapt along with the harmful bacteria, decreasing the likelihood that this resistance would develop, the researchers noted. With the sequence of the vampire bacteria genome in hand, researchers will be able to better understand how it seeks out and attacks specific kinds of bacterial prey. This could, eventually, enable the scientists to tailor-make vampire bacteria to attack just a single type of disease causing bacteria.
"It is possible that a living antibiotic such as M. aeruginosavorus — because it so specifically targets certain pathogens — could potentially reduce our dependence on traditional antibiotics and help mitigate the drug-resistance problem we are now facing," Wu said.
The study was published Sept. 21 in the journal BMC Genomics.
You can follow LiveScience staff writer Jennifer Welsh on Twitter @microbelover. Follow LiveScience for the latest in science news and discoveries on Twitter @livescience and on Facebook.
Jennifer Welsh is a Connecticut-based science writer and editor and a regular contributor to Live Science. She also has several years of bench work in cancer research and anti-viral drug discovery under her belt. She has previously written for Science News, VerywellHealth, The Scientist, Discover Magazine, WIRED Science, and Business Insider.










