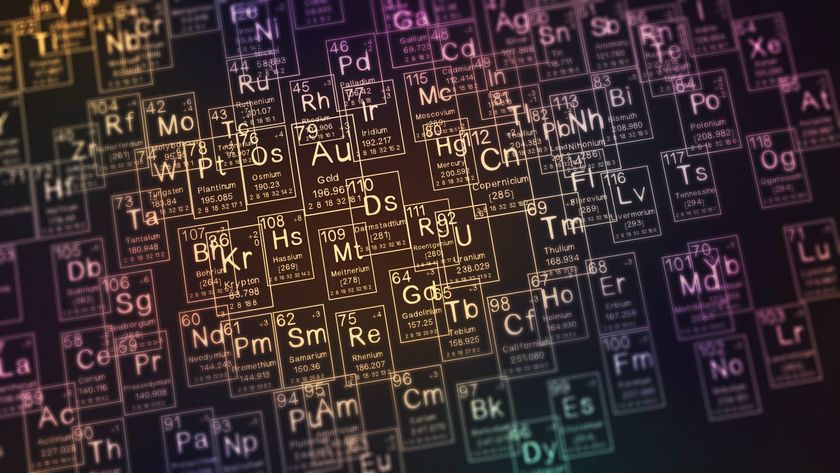
2 New Elements on Periodic Table Get Names

Two of the heaviest elements on the periodic table were officially named on Thursday (May 31).
The man-made elements 114 and 116, which contain 114 and 116 protons per atom, respectively, are now officially called flerovium (Fl) and livermorium (Lv).
The names were chosen to honor the laboratories that first created the elements: the Flerov Laboratory of Nuclear Reactions in Dubna, Russia, and Lawrence Livermore National Laboratory in Livermore, Calif.
Scientists at the two institutions collaborated to synthesize both of these heavy elements by smashing calcium, which has 20 protons, into curium, which contains 96 protons. When these atomic nuclei collided (the electrons were stripped off beforehand, rendering the atoms into ions), they glommed together to create element 116.
Such large "super-heavy" elements are not stable, so element 116 decayed almost immediately into element 114. In separate trials, the researchers created 114 independently by slamming together calcium and plutonium, which has 94 protons. [Graphic: Nature's Tiniest Particles Explained]
The elements were first made more than 10 years ago, but subsequent testing was required to confirm the fleeting elements' existence. The elements' official names were not approved until now by the International Union of Pure and Applied Chemistry (IUPAC), which governs chemical nomenclature.
Before they got their official names, flerovium and livermorium were temporarily called ununquadium and ununhexium, roughly based on the Latin words for the numbers 114 and 116. Four other super-heavy elements — 113, 115, 117 and 118 — have the temporary names of ununtrium, ununpentium, ununseptium, and ununoctium, and are waiting for their permanent monikers.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"These names honor not only the individual contributions of scientists from these laboratories to the fields of nuclear science, heavy element research, and superheavy element research, but also the phenomenal cooperation and collaboration that has occurred between scientists in these two countries," Bill Goldstein, associate director of Lawrence Livermore lab's Physical and Life Sciences Directorate, said in a statement.
The Flerov lab is named for Georgiy N. Flerov (1913-1990), a Russian pioneer of heavy-ion physics who discovered the spontaneous fission of uranium. The Lawrence Livermore lab was founded by E.O. Lawrence (1901-1958), an American physicist and Nobel laureate who already has an element, Lawrencium, or element 103, named after him.
In addition to helping discover flerovium and livermorium, scientists at Lawrence Livermore National Lab were collaborators on the projects that first created elements 113, 115, 117 and 118.
The creation of heavier and heavier elements is important not just for its novelty, but for the chance that soon scientists will find an "island of stability," an undiscovered region in the periodic table where heavy elements become stable again. If very heavy elements could exist for longer than nanoseconds, researchers hope they could experiment on them, and develop uses for them.
In 2011, three other new super-heavy elements, 110, 111 and 112, were officially christened darmstadtium (Ds), roentgenium (Rg) and copernicium (Cn), after the German city of Darmstadt, where they were created, as well as the German physicist Wilhelm Conrad Roentgen and the Polish astronomer Nicolaus Copernicus.
You can follow LiveScience senior writer Clara Moskowitz on Twitter @ClaraMoskowitz. For more science news, follow LiveScience on twitter @livescience.