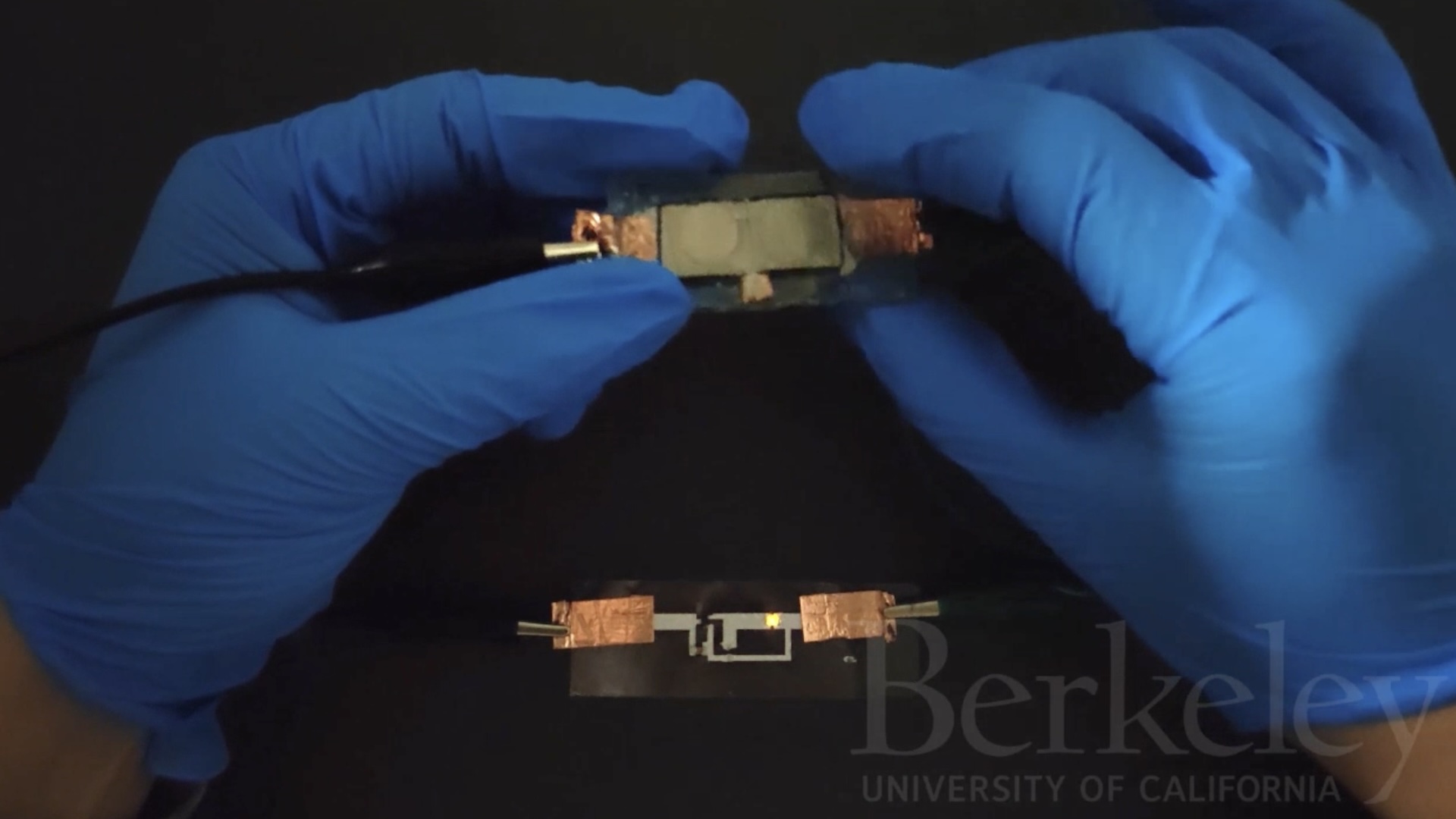
Laser Helps Measure Brain Activity

(ISNS) -- European researchers have developed a new tool for studying nerve cells in the brain. The implanted tool can simultaneously inject fluid into individual cells, shine light on them, and record their electrical activity.
The researchers demonstrated the value of the device, called an optrode, in experiments on mice. Laser pulses allowed them to influence the activity of nerve cells in the rodents' brains in a controlled manner.
"Proof of concept has been achieved," said Thomas Stieglitz, of the Laboratory for Biomedical Microtechnology at the University of Freiburg in Germany.
Stieglitz's team is one of several participating in the new field of optogenetics. It involves inserting genes from certain types of algae into other organisms, such as mice, to make the cells of those organisms responsive to light. Scientists can then influence the cells' electrical activity in a controlled manner by shining pulses of different colors of laser light onto them.
The team reported that its implant was the first multiple-use device to record the activity of single brain cells onto which it had transmitted light.
The team used a technique called transfection to insert genetic material from one organism into another. The optrode monitors the transfected cells for electrical activity as well as providing a channel for the laser light.
This new technology "has the potential to revolutionize the fields of neuroscience and neuroprosthetics," the researchers reported earlier this year in the journal Lab on a Chip.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"Optogenetics facilitates the science of investigating the behavior of nerve cells and fundamental research to better understand neural networks and brain behavior," said Stieglitz. "Scientists can use optogenetic experiments to study brain behavior and function – in anxiety disorders, for example."
Scientists and engineers from Freiburg and the Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland, worked together to create the device.
"Scientists need knowledge of genetic engineering to design 'shuttles' – the so-called vectors – for nerve cell transfection. This is the job of biologists," said Stieglitz. "In addition, engineers are sometimes asked to develop tools to optically stimulate the transfected cells and to record electrical nerve activity. The challenge is to develop the optrodes that combine electrical and optical activity."
One broad area where the device may be used is improving understanding of anxiety, depression, and motivation. Stieglitz's group aims to do that by applying its technology to networks of cells in the hippocampus, the part of the brain responsible for memory, and nuclei, which show up as gray matter. They will carry out the research in experimental animals.
"We will transfect cells that are candidates to malfunction in these disorders, and perform studies to modulate cell behavior by optical stimulation to understand the fundamental mechanisms," said Stieglitz.
The device, unlike current tools in optogenetics, combines all the required components into a single, self-contained device. This means only a single surgery is needed to implant the probe in an experimental animal, unlike some optogenetic devices, which require multiple surgeries.
The material that the team used to create the probe confers other advantages.
"It is made out of polymers only, plus a little bit of thin-film metal," said Stieglitz. "Polymers are more flexible than silicon in general and can follow the movements of the brain better because of that flexibility."
Previous studies had established the safety of the polymers for use in implantation in the nervous system.
David Lyon, assistant professor of anatomy and neuroscience at the University of California, Irvine School of Medicine, pointed out another advance achieved by the device. "A novel feature is the mechanism for delivering fluids through the chronically implanted optrode," said Lyon.
"The fluidic channel allows precise injection of the vector-carrying fluid," said Stieglitz.
The device also has the advantage of minute size. Its tip is only a quarter of a millimeter wide and a one-tenth of a millimeter thick.
However, Lyon, who is starting up an optogenetic research group, pointed out one disadvantage of the new optrode: It needs to be implanted semi-permanently to be most effective.
"You don't want an implant in the brain for several weeks," Lyon said.
The risk is that the implant can influence brain activity by its presence over a period of time.
One of the Freiberg-Basel team's goals for a second version of its optrode is an injection channel that dissolves over time. That would reduce the probe's size significantly.
"We also plan to have better integration of connectors to light, electrical plugs, and fluids to provide superior handling properties and to allow for use in really freely moving animals," said Stieglitz.
A former science editor of Newsweek, Peter Gwynne is a freelance science writer based in Sandwich, Massachusetts.
Inside Science News Service is supported by the American Institute of Physics.