Facts about iron
Discover the properties, sources and uses of the element iron.

From being a crucial building block of steel to nourishing plants and helping carry oxygen in your blood — iron is always busy helping sustain life on Earth.
Iron is a brittle, hard substance, classified as a metal in Group 8 on the Periodic Table of the Elements. The most abundant of all metals, its pure form rapidly corrodes from exposure to moist air and high temperatures. Iron is also the fourth most common element in Earth's crust by weight and much of Earth's core is thought to be composed of iron. Besides being commonly found on Earth, it is abundant in the sun and stars, according to the Los Alamos National Laboratory. Iron is crucial to the survival of living organisms, according to Jefferson Lab. In plants, it plays a role in the production of chlorophyll. In animals, it is a component of hemoglobin — a protein in blood that carries oxygen from the lungs to the tissues in the body.
Ninety percent of all metal that is refined these days is iron, according to the Royal Society of Chemistry. Most of it is used to make steel — an alloy of iron and carbon — which is in turn used in manufacturing and civil engineering, for instance, to make reinforced concrete. Stainless steel, which contains at least 10.5 percent chromium, is highly resistant to corrosion. It is used in kitchen cutlery, appliances and cookware such as stainless steel pans and skillets. The addition of other elements can provide steel with other useful qualities. For instance, nickel increases its durability and makes it more resistant to heat and acids; manganese makes it more durable, whereas tungsten helps it maintain hardness at high temperatures, according to Jefferson Lab.
Just the facts
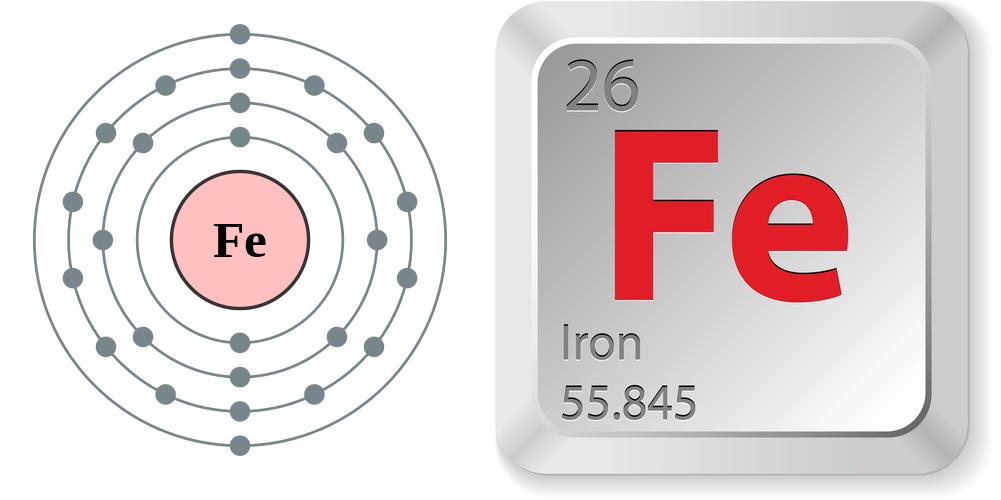
- Atomic number (number of protons in the nucleus): 26
- Atomic symbol (on the Periodic Table of Elements): Fe
- Atomic weight (average mass of the atom): 55.845
- Density: 7.874 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 2,800.4 degrees Fahrenheit (1,538 degrees Celsius)
- Boiling point: 5,181.8 F (2,861 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): (include how many are stable isotopes): 33 Stable isotopes: 4
- Most common isotopes: Iron-56 (natural abundance: 91.754 percent)
History and properties of iron
Archeologists estimate that people have been using iron for more than 5,000 years, according to Jefferson Lab. In fact, it turns out that some of the most ancient iron known to humans literally fell from the sky. In a study published in 2013 in the Journal of Archeological Science, researchers examined ancient Egyptian iron beads that date to around 3200 B.C. and found that they were made from iron meteorites. The Old Testament in the Bible also mentions iron multiple times, according to Los Alamos National Laboratory.
Iron is mostly obtained from minerals hematite and magnetite. In smaller degrees, it can also be obtained from the minerals taconite, limonite and siderite, according to Jefferson Lab. Iron has four different allotropic forms, which means that it has four different structural forms in which atoms bond in different patterns, according to Los Alamos National Laboratory. Those forms are called ferrites, known as alpha (which is magnetic), beta, gamma and omega.
Iron is an important nutrient in our diet. Iron deficiency, the most common nutritional deficiency, can cause anemia and fatigue that affects the ability to perform physical work in adults. It can also impair memory and other mental function in teens, according to the Centers for Disease Control and Prevention. Women who have iron deficiency while pregnant are at an increased risk of having small and early babies, the CDC warns.
There are two types of dietary iron: heme iron and non-heme iron. Heme iron — which is the more readily absorbed type of iron — is found in meat, fish and poultry, whereas non-heme iron — which is also absorbed but to a lesser extent than heme iron — is found in both plant foods (such as spinach, kale and broccoli) and meat, according to the American Red Cross. People absorb up to 30 percent of heme iron, compared with 2 to 10 percent of non-heme iron, the ARC reports, adding that foods rich in vitamin C such as tomatoes or citrus fruits can help absorb people absorb non-heme iron.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Who knew?
- Blood is red because of the interaction between iron and oxygen, according to the University of California, Santa Barbara. The blood looks red because of the way in which the chemical bonds between the two elements reflect light.
- Pure iron is actually soft and malleable, according to the University of Denver.
- In 2007, researchers discovered a huge plume of iron-rich water emanating from hydrothermal vents in the southern Atlantic Ocean.
- Iron is necessary for the growth of phytoplankton — tiny marine bacteria that use carbon dioxide from the atmosphere to fuel photosynthesis. Some researchers have therefore argued that fertilizing the oceans with extra iron could help suck up excess carbon dioxide. But a study published online in November 2010 in the Proceedings of the National Academy of Sciences found that this might not be such a good idea, as all this extra iron could actually trigger the growth of toxin-producing algae that contribute to the contamination of marine wildlife.
- About 90 percent of all metal that is refined today is iron, according to the Royal Society of Chemistry.
- Iron is a crucial component of a meteorite class known as siderites, according to Los Alamos National Laboratory.
- An iron pillar dating to about A.D. 400 still stands today in Delhi, India, according to Los Alamos National Laboratory. The pillar is about 23.75 feet (7.25 meters) high and measures 15.75 inches (40 centimeters) in diameter. Despite being exposed to weather conditions, the pillar has not corroded much due to its unique composition of metals.
- Examples of iron-rich foods include meat, such as beef, turkey, chicken and pork; seafood, such as shrimp, clams, oysters and tuna; vegetables, such as spinach, peas, broccoli, sweet potatoes and string beans; bread and cereals, such as bran cereals, whole wheat bread and enriched rice; other foods, such as beans, lentils, tomato paste, tofu and molasses, according to the American Red Cross.
- The surface of Mars is red due to a large amount of iron oxide (rust) on its surface, according to Nature. Mars has more than twice as much iron oxide in its crust than Earth.
- Earth's solid inner and liquid outer cores are primarily composed of iron (approximately 85 percent and 80 percent by weight, respectively). The electric current generated by the liquid iron creates the magnetic field protecting Earth, according to NASA. Iron is also found in the cores of all of the planets in the Solar System.
- Iron is the heaviest element formed in the cores of stars, according to JPL. Elements heavier than iron can only be created when high mass stars explode (supernovae).
- The Latin name for iron is ferrum, which is the source of its atomic symbol, Fe.
- The word iron is from an Anglo-Saxon word, iren. The word iron is possibly derived from earlier words meaning "holy metal" because it was used to make the swords used in the Crusades, according to WebElements.
Current research
Iron has been the subject of numerous medical studies, some of which show that high levels of iron in the blood may in fact be linked to an increased risk of cardiovascular problems. "There is some research suggesting that people who have more ferritin in their blood system and markers of higher iron in the body may be more at risk in terms of some cardiovascular diseases," said Judith Wylie-Rosett, a professor at the department of epidemiology and population health and the department of medicine at Albert Einstein College of Medicine of Yeshiva University in New York. "And whether that's causing the risk or that's a biomarker for something else going on is unclear," Wylie-Rosett told Live Science. (Ferritin is a type of protein that stores iron, while the ferritin test measures the amount of iron in your blood.)
In a study of more than 1,900 Finnish men ages 42 to 60 years, published in 1992 published in the journal Circulation, researchers found a link between high levels of iron and increased risk of heart attack. In a more recent study, published online in January 2014 in the Journal of Nutrition, researchers found that heme iron, found in meat, increased the risk for coronary heart disease by 57 percent, but no such association was found between non-heme iron and coronary heart disease risk.
Interestingly, recent research has also linked the accumulation of iron in the brain to Alzheimer's disease. In a study published in August 2013 in the Journal of Alzheimer's Disease, researchers found that the amount of iron in the hippocampus — an area of the brain associated with the formation of memories — was increased and associated with tissue damage in the hippocampus area in people with Alzheimer's disease, but not in healthy older people.
"The accumulation of iron in the brain may be influenced by modifying environmental factors, such as how much red meat and iron dietary supplements we consume and, in women, having hysterectomies before menopause," study author Dr. George Bartzokis, a professor of psychiatry at the Semel Institute for Neuroscience and Human Behavior at UCLA, said in a statement.
Iron deficiencies have also been linked with depression, according to a 2017 study published in the Journal of Psychiatric Research by a group of Australian researchers who were trying to find a link between genetics, iron levels and depression, especially with teenagers. The researchers found that although there is a link between iron levels in the bloodstream and measure of depression, there is no evidence for a genetic relationship between the two. The researchers used data available from twin studies and looked at a variety of factors while comparing teenaged twins to adult twins. The link between iron levels and depression is most likely to be observed during periods of time when the body requires higher amounts of iron such as during growth spurts.
A 2017 article published in the European Journal of Nutrition by a research group from Iran described a study in which iron supplements were given to new, non-anemic mothers with postpartum depression (PPD). A group of 70 women began the double-blind trial one week after giving birth and PPD symptoms were compared six weeks later. The group taking the iron supplement experienced a significantly greater improvement of PPD symptoms than the group taking the placebo.
Additional reporting by Rachel Ross, Live Science Contributor
 Live Science Plus
Live Science Plus