Mystery Popped: Science of Bubbles Decoded
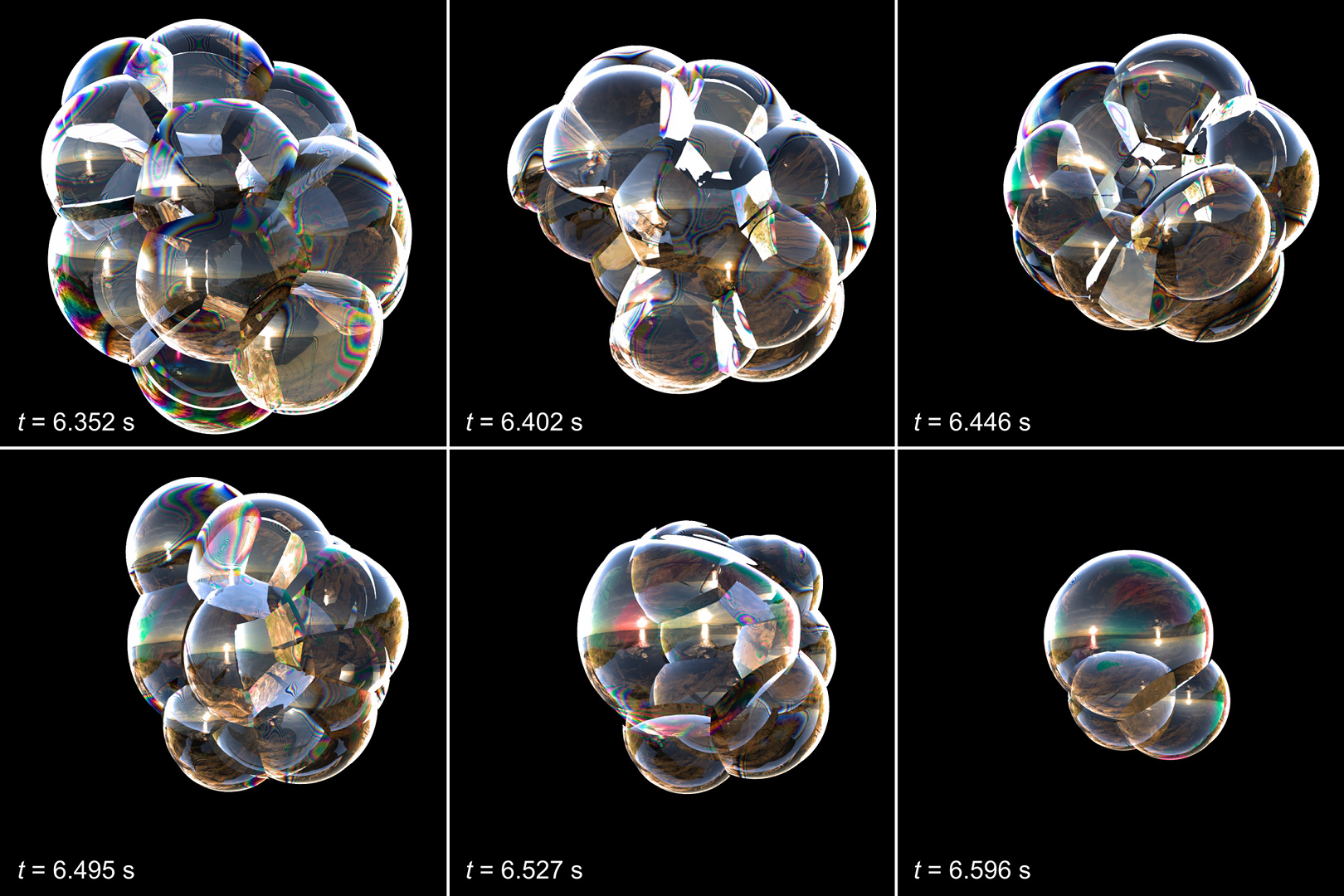
Anyone who has lathered up soap or seen frothy suds form on top of freshly poured soda has witnessed the delicate science of bubbles in action. But while bubbles and foamy materials are common in everyday life, scientists have struggled to model suds’ complicated behavior — the way clusters of bubbles grow, change shape and ultimately pop.
Now, researchers at the University of California, Berkeley have created a series of equations that model how foamy clusters evolve, based on their examination of shape-shifting soap bubbles. The findings, published today (May 9) in the journal Science, help predict the complex and dynamic movement of foams.
Understanding and predicting bubble behavior is important because the production of chemicals we rely on, such as flame-retardants, involves froths and foams.
Building mathematical models for foams is difficult because they are made of individual bubbles connected together in a cluster, often sharing walls or boundaries, said James Sethian, a professor of mathematics at the University of California, Berkeley and co-author of the new study.
"Physical effects drive these interfaces around, and the complexity has to do with the fact that the mechanics occur on a wide range of time and space scales," Sethian told LiveScience. "It's challenging to build numerical models that allow you to couple these wildly different scales together so that they talk to each other in a way that's accurate and physically reasonable." [Liquid Sculptures: Dazzling Photographs of Falling Water]
Sethian and his co-author, Robert Saye, identified three key phases of foam evolution: The rearrangement of the bubbles; the drainage of liquid through the bubbles' thin walls, or membranes; and the subsequent stage where the membranes become so thin the bubbles burst.
The researchers' tested out their model on different-size clusters of soap bubbles, and found the models accurately predicted the movement of the suds.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"The dynamics change as a function of the number of bubbles, the materials involved and the viscosity of the liquids," Sethian said.
Denis Weaire, a physicist and professor emeritus at Trinity College Dublin in Ireland, called the research "a fresh start" in the study of foam physics. Weaire was not involved in the new study, but penned an editorial article discussing the implications of the findings.
"I think people like me have been waiting for this development for quite a while," Weaire told LiveScience.
Bubbles and foams are created by trapping air pockets in liquids, and are dependent on a fluid property called surface tension. High surface tension is what enables a paperclip to float on the surface of water rather than being submerged.
When water flows from a tap, small bubbles are formed but pop very quickly. This is because the surface tension of water is high, so the bubbles develop very thin membranes, which cause them to easily rupture.
Surface active substances, or surfactants, are organic compounds that stick to the surface of water, which lowers the surface tension and stabilizes bubbles. Soap and dishwasher fluid are examples of materials containing surfactants, which explains why soapy water can create big clusters of bubbles, while normal water cannot.
Weaire said the new equations will help physicists study so-called unstable foams, in which various factors, such as gravity, cause fluids to drain through the bubbles' membranes, which eventually makes them burst.
"The challenge in the future will be to describe these dynamic situations, or unstable foams that are far from equilibrium," Weaire said. "Where it will all lead is hard to say, but this opens up a new center for the subject."
Follow Denise Chow on Twitter @denisechow. Follow LiveScience @livescience, Facebook & Google+. Original article on LiveScience.com.

Denise Chow was the assistant managing editor at Live Science before moving to NBC News as a science reporter, where she focuses on general science and climate change. Before joining the Live Science team in 2013, she spent two years as a staff writer for Space.com, writing about rocket launches and covering NASA's final three space shuttle missions. A Canadian transplant, Denise has a bachelor's degree from the University of Toronto, and a master's degree in journalism from New York University.