How do we know how old Earth is?
By measuring radioactive elements in rocks from Earth and other parts of the solar system, scientists can develop a timeline of our planet's early years.
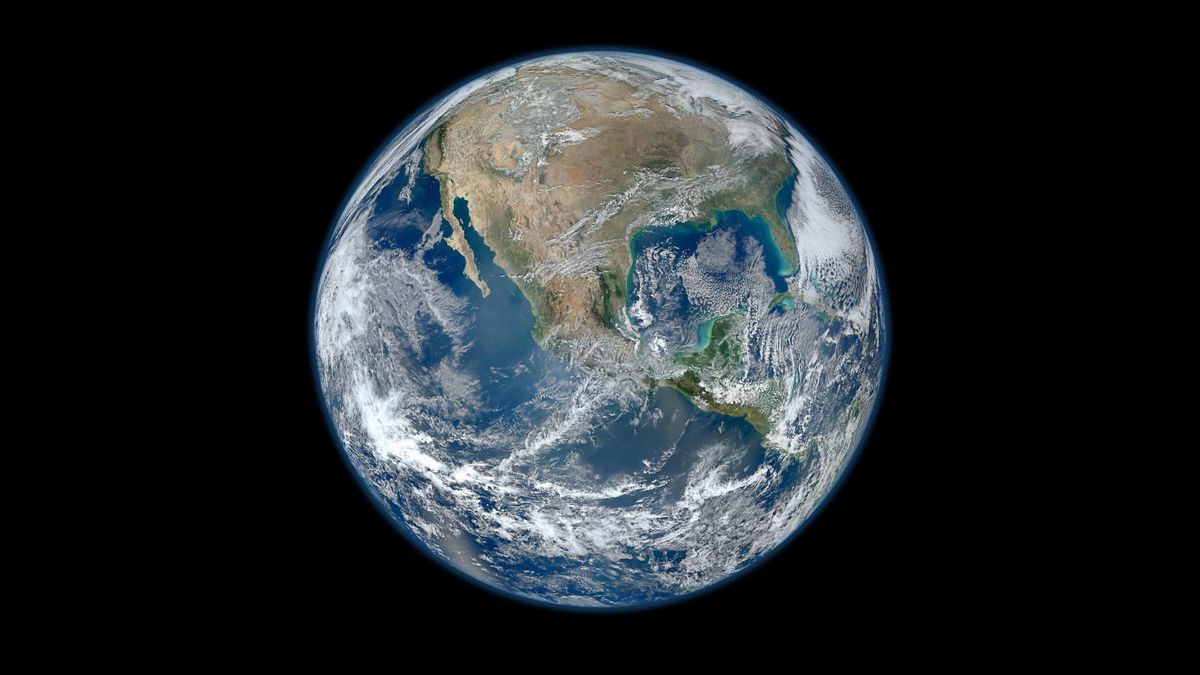
Earth is roughly 4.54 billion years old. In that time, it has seen continents form and disappear, ice caps expand and retreat, and life evolve from single-celled organisms into blue whales.
But how do we know Earth's age? We start by looking inside it.
"When you're an Earth scientist who looks at a rock, it's not just a rock; it's like that rock has a story that you can try to decipher," said Becky Flowers, a geologist at the University of Colorado Boulder.
When minerals form out of magma or lava, they often contain traces of radioactive material, such as uranium. Over time, those radioactive elements decay, meaning they spew radiation, eventually transforming them into new, more stable elements that remain trapped inside the mineral.
Take radioactive uranium-238, a common form of uranium. Its atoms will release energy until they eventually turn into lead. That process occurs at a fixed rate known as a half-life, which corresponds to the amount of time it takes for half of the atoms to decay. The half-life of uranium-238 is more than 4 billion years, meaning it takes more than 4 billion years for half of the uranium-238 in a sample to become lead. This makes it perfect for dating objects that are very, very old.
By knowing these half-lives, we can calculate how old a rock is based on the ratio of the "parent" radioactive element and the "daughter" stable element — a method called radiometric dating.
Related: How do scientists figure out how old things are?
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
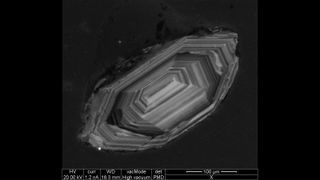
The mineral zircon is commonly used for radiometric dating because it contains a relatively large amount of uranium, Flowers said. Uranium-lead dating is just one type of radiometric dating. Other types use different elements; for example, radiocarbon dating, one of the most common methods, uses a radioactive isotope of carbon that has a half-life of thousands of years and is useful for dating organic matter.
Using these methods, geologists have found minerals on Earth that date as far back as 4.4 billion years, meaning the planet has been around at least that long. But if scientists say Earth is more than 4.5 billion years old, where did those extra 100 million years or so come from?
Earth, as mentioned, has changed a lot over billions of years, especially through processes such as plate tectonics, which shift the crust, birthing new land out of magma and subducting old land back underground. As a result, rocks from the very beginning of the planet's history are hard to find; they've long since eroded or melted back into raw material.
But scientists can use radiometric dating to determine the age of rocks from other parts of the solar system, too. Some meteorites contain materials that are more than 4.56 billion years old, and rocks from the moon and Mars have also been dated to around 4.5 billion years ago.
Those dates are pretty close to the time scientists think the solar system started to take shape out of the cloud of gas and dust surrounding the newborn sun. And by knowing all of these relative ages, we can start to piece together a timeline of how Earth, the moon, Mars and all of the other little rocks floating around in nearby space started to form.
Yet the transition from primordial dust cloud to planet Earth didn't happen all at once but rather over millions of years, Rebecca Fischer, an Earth and planetary scientist at Harvard University, told Live Science. That means our understanding of Earth's age will always be less about a specific year when the planet formed and more about a general sense of the era when our home planet started to take shape.

Ethan Freedman is a science and nature journalist based in New York City, reporting on climate, ecology, the future and the built environment. He went to Tufts University, where he majored in biology and environmental studies, and has a master's degree in science journalism from New York University.