Inside Life Science: Sticky Stem Cells
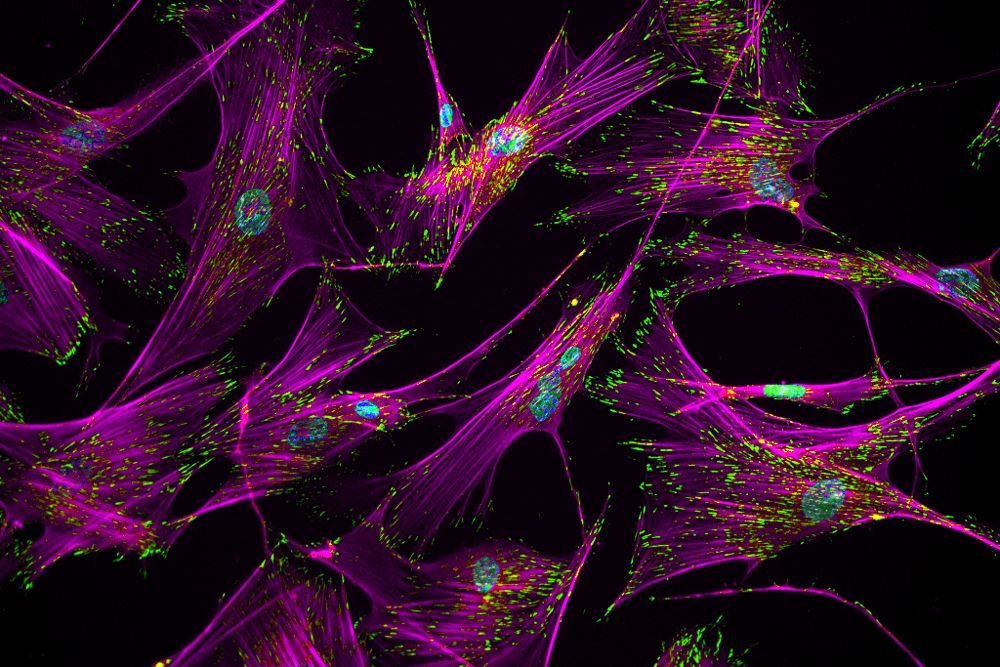
Imagine a group of barnacles hanging onto a rock against a relentless ocean current. That's not unlike what's happening to the human cells in this image. Adhesive complexes containing actin stress fibers, stained magenta, and the protein vinculin, stained green, help influence how strongly cells adhere to surfaces such as the walls of blood vessels or bone, or in the case of this picture, a glass slide.
Researchers used the meeting point of these two molecules, known as focal adhesions, to develop a new way of isolating human induced pluripotent stem cells.These cells are specialized tissue or organ cells that have been reprogrammed into a stem cell-like state and can become just about any cell type. Understanding and harnessing this cellular reprogramming could aid the development of therapies for replacing defective or diseased cells.
One time-consuming challenge is the process of separating the stem cells from the other cells in a culture. Less than one percent of the starting human cells become reprogrammed to iPS cells. Isolating them is akin to finding the proverbial needle in a haystack — only these needles are cell colonies that can be easily damaged or destroyed.
Using information about how strongly different cells stick to surfaces, a research team led by scientists at the Georgia Institute of Technology has developed a faster, more efficient way of collecting human iPS cells.
The method, called micro stem cell high-efficiency adhesion-based recovery (μSHEAR), uses a microfluidic device to which cells, including the human iPS cells, adhere well. Researchers expose the cells attached to the device to the flow of a fluid. Like the barnacles on the rock, the iPS cells hang onto the device, while others are swept away.
The technique, which takes only 10 minutes to perform, results in a greater than 95 percent pure human iPS cell culture in which the cells remain viable. In addition, since iPS cells' adhesion strengths change as they are reprogrammed, scientists can isolate cells at different stages simply by modifying the rate of fluid flow.
The researchers predict that the method could be scaled up, allowing scientists to experiment with a greater number of cells at a time and thereby speeding progress toward potential medical therapies. The ability to isolate iPS cells at different stages of reprogramming may also help researchers answer fundamental questions about how the cells undergo reprogramming.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The research reported in this article was funded in part by the National Institutes of Health under grants R01GM065918, R43NS080407 and RC1CA144825.
Learn more:
The Forces That Bind from Findings
Also in this series:
This Inside Life Science article was provided to LiveScience in cooperation with the National Institute of General Medical Sciences, part of the National Institutes of Health.












