Prescription Lockers Recalled by Locker
The U.S. Consumer Product Safety Commission, in cooperation with Locker Brand Inc. of Henderson, Nev., announced a voluntary recall of about 59,600 Medicine Bottle Storage Containers.
Hazard: The medicine container can open by applying pressure to the latch when it is locked. This could result in unauthorized access to medicine bottles in the container.
Incidents/Injuries: None reported
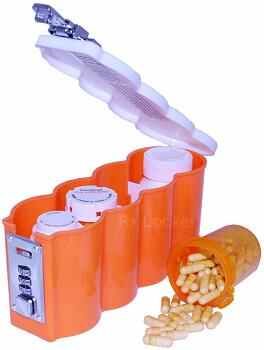
Description: The Rx Locker is a medicine bottle storage container. It is made of orange plastic with a white top resembling four pill containers. It has a metal, three-number combination lock on the front. Production batch numbers are printed on the bottom of the container. The recalled containers were manufactured between May 2010 to December 2010 and can be identified by the last six numbers in the production batch number. Recalled batch numbers end with: 052 010, 062 010, 082 010 and 122 010.
Sold at: Bed Bath & Beyond, CVS, Walgreens and the Locker Brand website from June 2010 through October 2011 for about $15.

Manufactured in: China.
Remedy: Consumers should immediately stop using this product and contact Locker Brand for a free return mailer. When the product is returned, Rx Locker will provide a full refund.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Consumer Contact: For additional information, contact Locker Brand toll-free at (888) 491-6617 between 9 a.m. and 5 p.m. PT Monday through Friday, e-mail the firm at admin@lockerbrand.com, or visit the firm’s website at www.rxlocker.com.