Introvale Oral Contraceptive Recalled
June 5, 2012 - Sandoz is conducting a voluntary recall of 10 lots of its generic oral contraceptive Introvale® in the US, following a recent report of a packaging flaw.
The probability of this packaging flaw causing serious adverse health consequences is remote and Sandoz is not aware of any reports of related adverse events. This recall is being undertaken as a precautionary measure to minimize any potential of patients being impacted. The recall is being conducted with the knowledge of the Food and Drug Administration.
The lot numbers involved in the recall are as follows: LF00478C, LF00479C, LF00551C, LF00552C, LF00687C, LF00688C, LF00763C, LF00764C, LF00765C and LF01261C. These lots were distributed only in the US between January 2011 and May 2012.
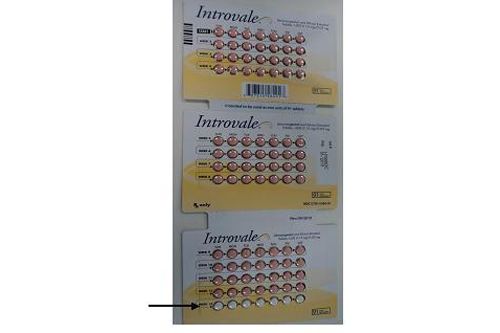
The recall was decided after a consumer reported that the white placebo tablets were mistakenly in the ninth row (labeled "Week 9") of the 13-row blister card, rather than in the correct position in the 13th and final row (labeled "Week 13"). Each three-month blister card contains 84 peach-colored active tablets and seven white placebo tablets in 13 rows, each representing one week (see figure below). While the white placebo tablets can be clearly distinguished from the peach-colored active tablets, the risk of an unintended pregnancy for a patient taking the wrong tablet over several days cannot be excluded.
In the unlikely event that a patient finds a white placebo tablet in any position other than the 13th and final row (Week 13), they should immediately begin using a non-hormonal form of contraception. They should also immediately contact their healthcare professional as well as Sandoz to report the finding via the Sandoz Drug Information Direct Line at 800-525-2492, 24 hours/day, seven days a week, or via email at qa.druginfo@sandoz.com.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.