Facts About Antimony
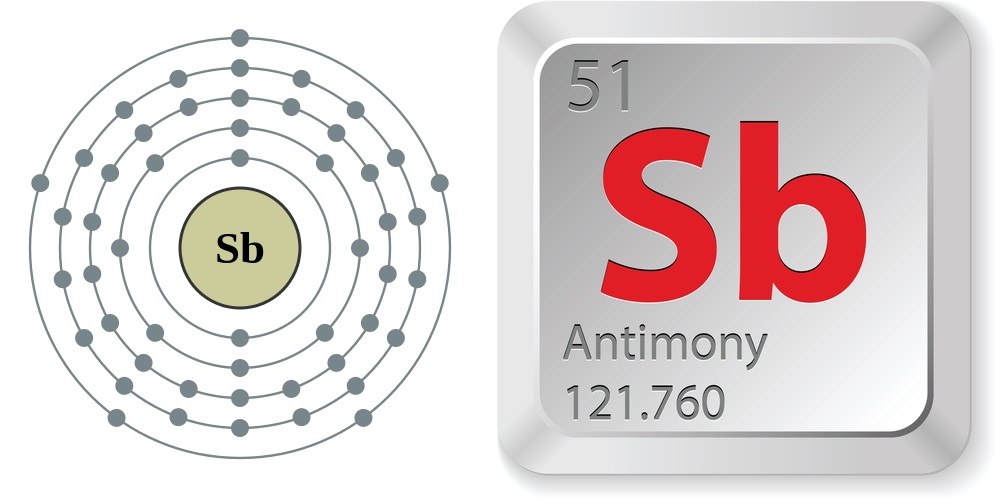

Word origin: Antimony was named after the Greek words anti and monos to mean “a metal not found alone.” The chemical symbol, Sb, comes from the element's historical name, stibium.
Discovery: Antimony was a known metal in the 17th century and was likely used even earlier.
Properties of antimony
Antimony is a silvery, lustrous gray metal. It is in the metalloid group of elements. It is a poor conductor of heat and electricity. The metal and its compound forms can be toxic. [See Periodic Table of the Elements]
Sources of antimony
Antimony is a rare element but can sometimes be found naturally. However, it’s mostly in the form of its sulfide stibnite.
Uses of antimony
The pure form of antimony is used to make certain types of semiconductor devices, such as diodes and infrared detectors. An alloy of lead and antimony is used in batteries, low friction metals, small arms and tracer bullets, cable sheathing as well as other products. Other compounds of antimony are also used to make paints, glass, pottery and ceramics.
(Source: Los Alamos National Laboratory)
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.











