Facts About Xenon

Pronounced "ZEE-non," this element is a gas primarily used in light manufacturing. Xenon is one of the inert or noble gases and is odorless, colorless, tasteless and chemically non-reactive. While not toxic on its own, its compounds are strong oxidizing agents that are highly toxic.
Just the facts
According to the Jefferson National Linear Accelerator Laboratory, the properties of helium are:
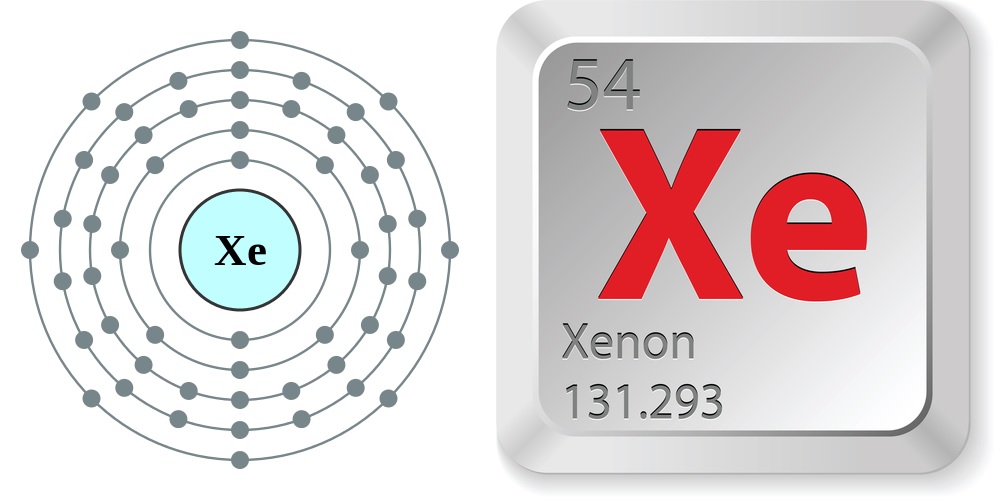
- Atomic number: 54
- Atomic weight: 131.293
- Boiling point: 165.03 K (-108.12°C or -162.62°F)
- Melting point: 161.36 K (-111.79°C or -169.22°F)
- Phase at room temperature: Gas
- Density: 0.005887 grams per cubic centimeter
- Element classification: Non-metal
- Period number: 5
- Group number: 18
- Group name: Noble Gas
History
Xenon was discovered by Scottish chemist William Ramsay and English chemist Morris Travers in July 1898 at the University College London. This wasn't their first discovery. The pair already extracted argon, neon and krypton from liquid air.
Their discovery came about when a wealthy industrialist, Ludwig Mond, gifted the team a new liquid-air machine. With the new machine, they extracted more krypton from liquid air. Then, they repeatedly distilled the krypton and isolated a heavier gas. Ramsay and Travers examined the heavier gas in a vacuum tube and saw that it emitted a beautiful blue glow. They categorized the new gas as inert and called it xenon, derived from the Greek "xenos," which means stranger.
However, in 1962 Neil Bartlett proved that xenon was not, in fact, inert. It could cause reactions and compounds. He proved this by making a fluorine derivative. Since then, more than 100 xenon compounds have been made, according to the Royal Society of Chemistry.
Natural xenon has nine stable isotopes and 20 unstable isotopes. Some compounds that can be formed with xenon include difluoride, xenon deuterate, xenon trioxide, sodium perxenate, xenon hydrate, tetrafluoride and hexafluoride. Another interesting compound is a metallic xenon created by using massive amounts of pressure.
Sources
Xenon is a trace gas found in the Earth's atmosphere to the extent of about one part in 20 million, According to the Los Alamos National Laboratory. This makes it very rare. It is also found in Mars' atmosphere at 0.08 ppm.
Get the world’s most fascinating discoveries delivered straight to your inbox.
This noble gas can also be found down on Earth. Some mineral springs emit xenon. Companies obtain the gas for commercial use from industrial plants that extract the gas from liquid air.
Xenon may also be found in the Earth. For a long time, scientist suspected that 90 percent more of the gas should be found in the Earth's atmosphere, based on their knowledge of other noble gases. "The missing xenon paradox is a long-standing question," said Yanming Ma, a computational physicist and chemist at Jilin University in Changchun, China. [From: Missing Xenon Gas Found in Earth's Core].
Eventually, scientists, including Ma, found evidence that the missing gas may be found at the Earth's core. The extreme temperatures and pressures found in Earth's core may cause xenon to bond with iron and nickel located in the core, storing the gas there. "We do hope future high-pressure experiments can be carried out to confirm our predictions," Ma said.
Uses
Xenon creates a blue or lavender glow when subjected to an electrical discharge. Lamps that use xenon illuminate better than conventional lights. For example, stroboscopic lamps, photographic flash lamps, high-intensive arc-lamps for motion picture projection, some lamps used for deep-sea observation, bactericidal lamps, sunbed lamps and high-pressure arc all use this gas. In fact, you probably see xenon lamps on a regular basis. Some vehicle headlights use xenon. If you see headlights that give off a soft blue glow, they are probably made with xenon.
The gas has other uses, too. It is used in nuclear energy plants and for filling television and radio tubes. Silicon microprocessors are etched with xenon difluoride. Xenon ion propulsion systems keep some satellites and other spacecraft in orbit. Xenon is even used to manufacture a drug called 5-fluorouracil, which is used to treat certain types of cancer, according to the Royal Society of Chemistry.
Current research
There are several studies that focus on xenon. The Xenon Dark Matter Project, for example, is experimenting with a liquid xenon detector to search for dark matter. Dark matter is described as an invisible glue that holds the universe together. In this experiment, liquid xenon is put in a time projection chamber. When the particles in the chamber act in a way they shouldn't this may be a sign of dark matter interacting with the particle.
The Large Underground Xenon (LUX) collaboration is another, similar experiment. This dark matter detector also uses liquid xenon. Though the project didn't find anything, the research has reshaped ideas about dark matter.
Who knew?
- Radioactive iodine-131 can decay into stable xenon, as it did in Fukushima.
- Xenon isn't the only noble gas. Neon, argon, krypton, helium and radon are also noble gases.
- Like helium, you can fill balloons with xenon, but it is very expensive and the balloon becomes very heavy because the gas is so dense. An average balloon can hold around 40 lbs. (18.1 kilograms) of xenon, according to an experiment by the Royal Society of Chemistry.
- Xenon atoms added to liquid helium are used to observe quantum tornadoes.
Additional resources