Francium: Facts about the elusive radioactive element
Does francium have any practical uses?
Francium — the 87th element on the periodic table — is a naturally occurring, but incredibly rare, radioactive element. It forms and decays extremely quickly, so it has no practical uses, and it is mostly used in scientific research.
However, the element has some intriguing properties: It is one of the only elements with no known stable form, and it has the largest atomic radius.
Some elements on the periodic table are relatively abundant on Earth — hydrogen, helium, oxygen and carbon, for example — while others are far more elusive — promethium and thulium being the two most uncommon. Francium sits very much at the elusive end of the spectrum.
"Francium-223 forms naturally during the radioactive decay of other elements, but it is estimated that there is only about 30 grams [1 ounce] of francium in the entire crust of the Earth at any one time," Christopher Barnett, a postdoctoral chemistry researcher at the University of Sydney, Australia, told Live Science in an email.
The element was first found in 1939 by French physicist Marguerite Perey, a prodigy of Marie Curie, who named the new discovery after Perey's homeland. This scarce element is considered to be one of the last naturally occurring elements discovered on Earth.
Francium fast facts
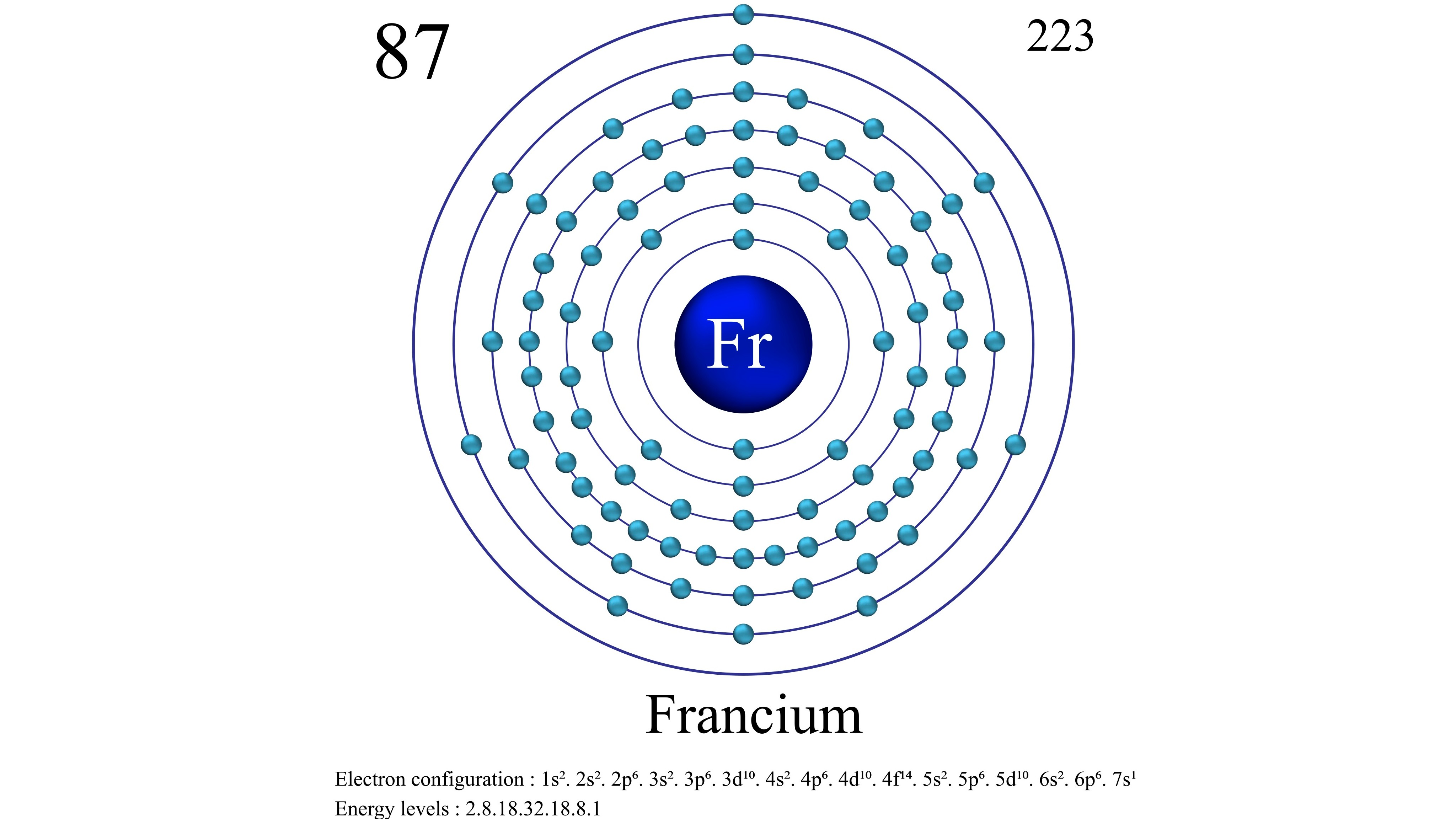
- Atomic number (number of protons in the nucleus): 87
- Atomic symbol (on the periodic table of elements): Fr
- Atomic weight (average mass of the atom): 223
- Density: unknown
- Phase at room temperature: solid
- Melting point: 80.6 degrees Fahrenheit (27 degrees Celsius)
- Boiling point: 1,250 F (677 C)
Uses and half-life
Because francium is so rare, scientists who want to study it first have to create it in nuclear reactions — either by “bombarding radium with neutrons, or thorium with protons," Barnett said.
Radium — atomic number 88 — is a radioactive, metallic chemical element, while thorium — atomic number 90 — is a naturally occurring radioactive metal.
So, is francium useful? Have scientists found any practical applications for it? The simple answer is "no."
The most stable form of francium, according to Barnett, is francium-223, but even so "the half-life is so short that there are no known biological processes that use it," Barnett said.
A half-life is a measure of how long it takes half of a sample of radioactive material to decay."One might expect the decay [of any element] to happen linearly and at a constant rate," Barnett said. "However, we have found that the rate of decay changes with the amount of substance left, such that the amount of material will halve in a constant time period."
Francium has a half-life of 22 minutes. So, if observing a sample of francium, half of the original amount will be left after 22 minutes. After another 22 minutes, there will be a quarter of the initial amount. It decays into radium-223 through beta decay, when an electron is emitted, or astatine-219 through alpha decay, when an "atom's nucleus sheds two protons and two neutrons in a packet that scientists call an alpha particle," according to JLab.
"Francium's half-life is extremely short for a radioactive element. For comparison, technetium-99m (which is used in medical imaging) has a half-life of six hours. Uranium-235 (the type used in nuclear reactors) has a half-life of 703,800,000 year[s]," Barnett said.
While francium's half-life is incredibly short and though it is very toxic due to its radioactivity, its characteristics make it a compelling subject to study.
"Francium has some interesting properties. It is used to develop and test theoretical models, and I can see these being very useful to test machine-learning-based physics and chemistry models."
Barnett also noted that francium has never been observed with the naked eye because "there has never been enough isolated [francium] to know what it looks like." Barnett predicts that it will probably be "a silvery-grey metal," similar to other alkali metals, but admits "we just don't know for sure."
Is francium dangerous?
Given that francium can only be found in miniscule quantities, it doesn't pose much of a risk to humans, but what if, hypothetically speaking, someone was able to get hold of a large amount of it? Would francium be considered dangerous?
"There are two main risks with francium," Barnett said. "One is radioactivity. When it decays, it releases high energy particles that can ionise the surrounding tissues (causing burns), or break DNA strands (resulting in cancer).
Yet, because francium is so hard to make, getting enough to cause a serious issue would be unlikely. The most ever isolated was less than 0.000000001% of that found in a brand-new smoke detector. Given its much shorter half-life, most of that radiation would be emitted in a few hours, compared to years for a smoke detector.
"The other risk is that it is an alkali metal. It is — or rather would be, if we could get enough of it together — very reactive, and would likely catch fire most spectacularly."
Originally published on Live Science on Sept. 11, 2013, and rewritten on July 26, 2022.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

Joe Phelan is a journalist based in London. His work has appeared in VICE, National Geographic, World Soccer and The Blizzard, and has been a guest on Times Radio. He is drawn to the weird, wonderful and under examined, as well as anything related to life in the Arctic Circle. He holds a bachelor's degree in journalism from the University of Chester.