Monster Mash: Protein Folding Gone Wrong
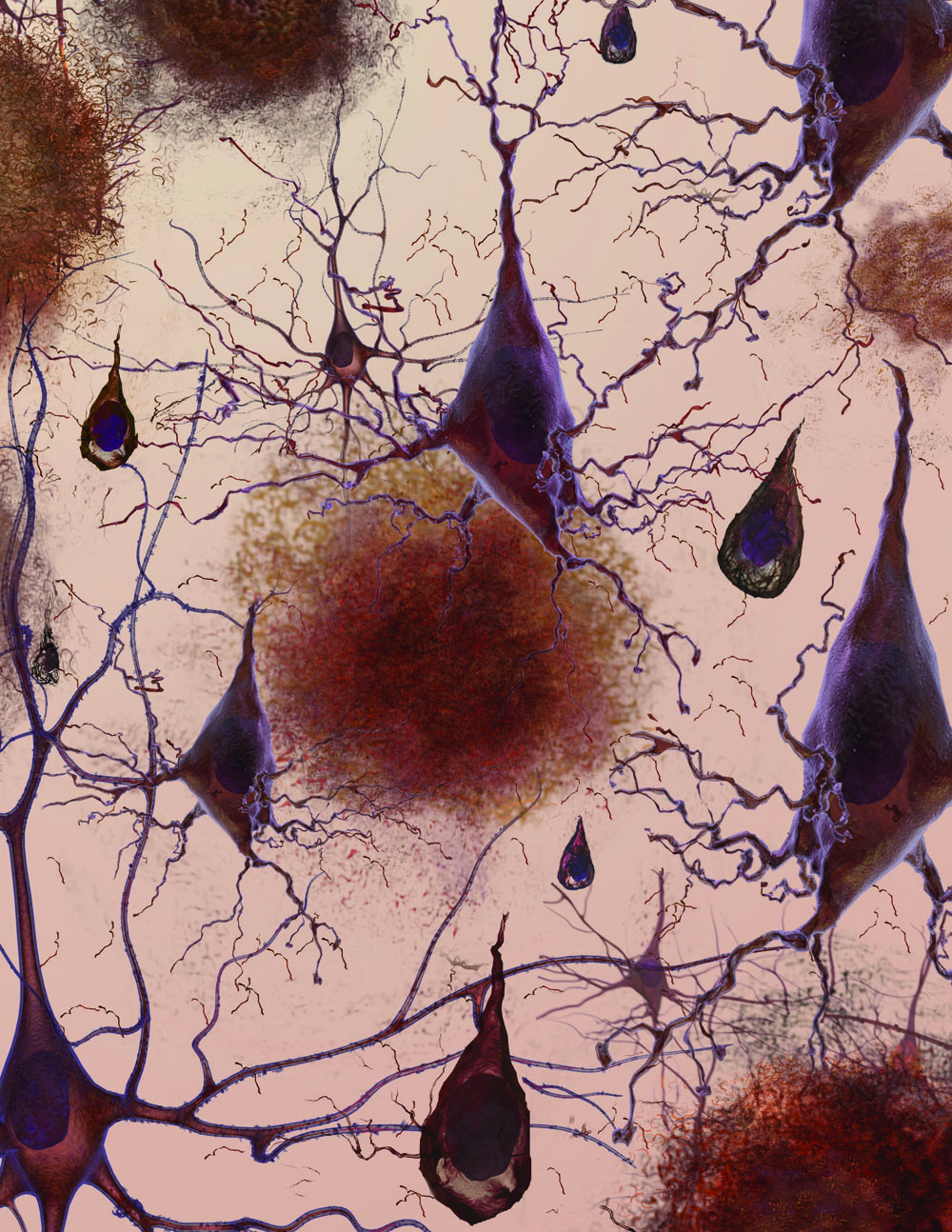
Imagine a 1950s horror movie monster — a creeping, gelatinous, gluey tangle of gunk that strangles everything around it. That's what amyloid plaques are like when they form in body tissues. These gooey protein clumps are associated with many chronic and debilitating disorders, including type 2 diabetes and neurodegenerative diseases like Parkinson's and Huntington's.
Amyloid plaques were a mystery for many years. The German physician Alois Alzheimer first noticed them in the early 1900s in the brain of a deceased patient who had experienced a peculiar form of memory loss and mood swings — symptoms of the disease that now bears his name. A few decades ago, scientists determined the basic structure of the plaques. Since then, researchers, many funded by the National Institutes of Health, have made enormous strides in understanding how these structures play roles in disease.
Misshapen Mess
In most healthy proteins, a chain of small molecules called amino acids folds up in a precise way. Proteins are built from combinations of long, straight coils; hinges; and wide, flat sections called beta sheets. All of these pieces have to be in the right places for a protein to carry out its unique function and avoid sticking to itself or to other proteins.
Amyloid plaques begin to form outside cells when a protein unfolds in response to a mutation or cellular stress like heat. While many proteins will refold into their healthy shapes, some will misfold. In amyloid-forming proteins, sections of amino acid chains that don't normally form beta sheets may rearrange themselves into this flat structure. When this happens, the beta sheets can pile on top of each other and stick together. Even only a few stacked beta sheets can be toxic: Like a vampire, they can pierce holes in cell membranes, causing the cells to die. Amyloid beta sheets can accumulate on one another almost endlessly, becoming long, cell-entangling threads called fibrils. Globs of many fibrils make the plaques that are the hallmark of Alzheimer's and similar diseases.
Keeping Away the Monsters
The endless formation of amyloid plaques is like a school dance gone very much awry.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Imagine a cell "prom." Most of the time, protein molecules swirl about in specific steps. Cells even have special proteins called chaperones that try to keep order. Chaperones perform various roles in helping proteins fold into and maintain their normal forms. One large chaperone complex, for example, can completely surround a protein that's unfolding, shield it from other proteins that might stick to it, and help it to properly refold.
All's well at the molecular dance until a grisly, amyloid-forming protein shows up. Scientists have learned that even one molecule of these proteins can cause healthy copies of the same protein to misfold and build gluey plaques. The misfolded proteins can spread by ingestion and even blood transfusions. Such infectious proteins, called prions, lead to Creutzfeldt-Jakob disease and bovine spongiform encephalopathy (also known as "mad cow" disease).
Too many amyloid proteins can overwhelm the chaperones, causing plaque formation to outpace the protective activities. Further research may reveal how to ward off this nightmare, potentially helping people who have or may develop amyloid-related diseases. Some possibilities being studied include using drugs to keep at-risk proteins properly folded or to increase the power or number of the cell's chaperone molecules.
This Inside Life Science article was provided to LiveScience in cooperation with the National Institute of General Medical Sciences, part of the National Institutes of Health.
Learn more:
Structural Biology Booklet, Fact Sheet and Video
Also in this series: