Holiday Snapshots: Seasonal Cells
Nestled snug while visions of ... cells danced in their heads?
What's red and green all over? While this time of the year may have you guessing a poinsettia, holiday garland or even a sunburned elf, another answer is: snapshots of cells.
Scientists use imaging techniques that harness light-emitting molecules and compounds to illuminate DNA, proteins and other targets of interest. By visualizing the targets' locations and tracking their movements, researchers can learn more about their role in fundamental life processes such as cell division and development. Common imaging tools include green fluorescent protein from jellyfish and red fluorescent protein from mushroom coral. When incorporated into a cell, these colorful molecular markers glow under harmless wavelengths of light.
Just in time for the holidays, we've wrapped up a few red and green cellular images from basic studies — many using model organisms that have operating systems similar to ours — that were funded by the National Institutes of Health.
Growing and Glowing

2013 BioArt Video Winners - Amanda L. Zacharias*† and John I. Murray* from FASEB on Vimeo.
To understand early development, scientists often turn to the embryos of C. elegans, a microscopic roundworm. Researchers have mapped out the developmental fate of each of the worm's cells — whether it will become part of the worm's mouth, intestine, nervous system or other organ. This time-lapse video shows the development of C. elegans, from a single cell to its final 959-cell stage.
In the video, cell nuclei are green and cells that have an active version of the gene ceh-27, which is required for proper embryonic development, are red. Like many molecules in C. elegans, ceh-27 has a human counterpart — a gene that, when altered, causes heart defects. The video, produced by Amanda L. Zacharias and John I. Murray of the University of Pennsylvania Perelman School of Medicine in Philadelphia, was a winner in the 2013 FASEB BioArt contest.
Spotting Errors in Cell Division
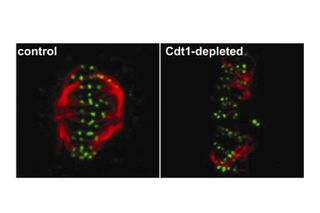
These pictures show two tales of cell division. Both cells are in metaphase, the stage when duplicated chromosomes align in the middle of the mitotic spindle. The spindle is a structure inside a cell's nucleus that divides genetic material between daughter cells. Rope-like microtubules (red) are attached to proteins at the ends of chromosomes (green).
The image on the left shows an intact spindle and the image on the right shows a collapsed one. A protein called Cdt1 helps the spindle keep a stable structure during metaphase; when it's missing, chromosomes can't divide evenly between new cells.
Insight into the role of Cdt1 could lead to new therapies for diseases associated with improper cell division, such as cancer.
Marking Heads or Tails
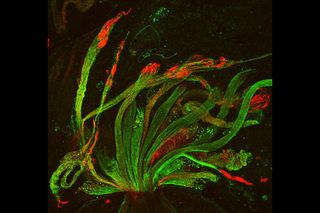
Developing spermatids — precursors of mature sperm cells — begin as small, round cells and mature into long-tailed, tadpole-shaped ones. In the sperm cell's head is the cell nucleus; in its tail is the power to outswim thousands of competitors to fertilize an egg.
As seen in this microscope image, fruit fly spermatids start out as bouquets of interconnected cells. A small lipid molecule called PIP2 helps spermatids tell their heads from their tails. Here, PIP2 (red) marks the nuclei and a cell skeleton-building protein called tubulin (green) marks the tails. When PIP2 levels are too low, some spermatids get mixed up and grow with their heads at the wrong end. Because sperm development is similar across species, studies in fruit flies could help researchers understand male infertility in humans.
Visualizing Protein Clumping
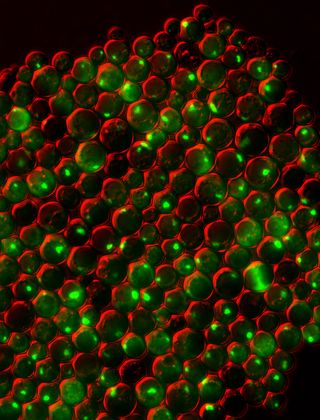
Protein clumping plays a role in many human diseases, including Parkinson's and Alzheimer's, so knowledge of why it happens — and what prevents it in healthy cells — could aid the development of treatments. To figure out what factors within cells cause proteins to misfold and stick together, scientists can use simple model organisms such as yeast.
This picture shows a group of yeast cells that are deficient in zinc, a metal that plays a key role in creating and maintaining protein shape. The cells also lack a protein called Tsa1, which normally keeps proteins from sticking together. Green areas highlight protein tangles caused by the double deficiency. Red, which is a false color generated by the imaging technique, outlines the cells.
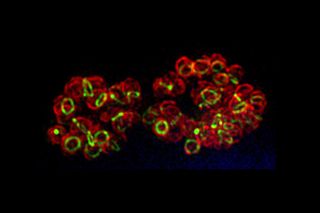
Revealing Bacterial Building Blocks

Many antibiotics, including penicillin and vancomycin, work by blocking bacteria from making a mesh-like polymer that gives structural strength to their cell walls. Researchers had suspected that this polymer, called peptidoglycan, also forms in C. trachomatis, a class of bacteria responsible for infections causing blindness, a sexually transmitted disease and childhood pneumonia. But scientists had not been able to confirm their suspicion until now.
With the aid of a new method that uses chemically modified peptidoglycan building blocks tagged with a fluorescent probe, researchers have finally seen peptidoglycan in the bacteria'scell wall. Here, peptidoglycanappears as lime-colored links inside red-stained bacterial cells. In addition to answering a long-standing question in microbiology, the imaging advance may aid the development of antibiotics for a range of infections.
This Inside Life Science article was provided to LiveScience in cooperation with the National Institute of General Medical Sciences, part of the National Institutes of Health.
See more:
Also in this series:
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.