The Little-Known Science That Improved Everything Around Us (Op-Ed)

This article was originally published at The Conversation. The publication contributed the article to Live Science's Expert Voices: Op-Ed & Insights.
UNESCO has declared 2014 as the International Year of Crystallography. But why? Quite simply because the science of crystallography has revolutionised how we live – and yet few people know about it.
Crystallography is the study of crystalline solids to understand how the atoms inside the solids are arranged. Normally this involves firing a beam of X-rays at a sample and recording the pattern of those scattered X-rays. From the interpretation of these patterns we can deduce information about the way atoms are arranged in a solid. By understanding the atomic arrangement we can interpret the properties these solids display and hopefully improve upon them. Single crystals (like a single grain of salt or sugar) scatter a single beam of X-rays as many well-divided beams that can be recorded as a series of spots on an X-ray sensitive plate.
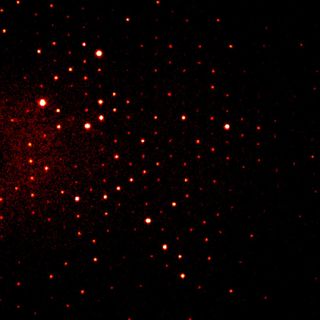
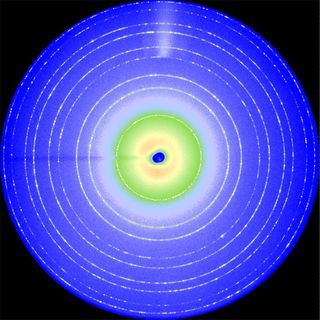
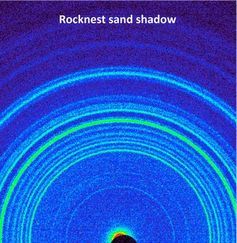
Powdered samples, for example, icing sugar or cement, scatter X-rays in cones that appear as rings on an X-ray sensitive plate. Interpretation of the X-ray scattering patterns from single crystals and powders is the realm of crystallography.
We may not recognise it but crystallography is fundamental to many branches of science and technology that we take for granted in our daily lives. Here is a story of my normal day, using which I’ll demonstrate its impact:
My alarm clock wakes me at 6:45am. I’m relieved that the house doesn’t seem to have blown down in the storm of last night. I make some coffee and check my emails on my phone. I have breakfast and then brush my teeth before going to work. The car starts first time and I drive to work, a university campus where lots of buildings have sprung up recently. The drive has given me a headache so when I get to my office I take two paracetamol tablets. Then it’s on with the day – my work as a crystallographer.
The batteries in my alarm clock and electric toothbrush, like most electronic devices, contain a complex crystalline material that allows the passage of an electric current. An enormous amount of research has gone into designing the materials for the task and at the heart of this is X-ray crystallography. Scientists have used this technique to understand and improve the lithium-ion conductors in batteries.
My house and work buildings rely on concrete and we take it for granted, but producing concrete is a really complex chemical reaction. The development of concrete has relied heavily upon crystallography – scientists have tweaked the mixture and used X-rays to understand how changes in composition lead to changes in atomic structure, which form the basis of cement’s strength and hardening.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The metals used in my car are examples of lightweight, strong alloys that are studied by crystallography. For example, the technique can distinguish between an appropriately cooled sample and one cooled in the wrong way (because the latter is more likely to crack). Crystallography has been used to study stress in materials and design components that resist stress. Without crystallography we simply wouldn’t have the lightweight components widely used today in cars and aeroplanes.
Similarly, the rise of microelectronics such as my phone and tablet would not have happened without crystallography. The complex semiconductors containing layers of different materials with matched structures were designed with the help of crystallography.
And what of the paracetamol that I took for a headache? The impact of crystallography in the pharmaceutical industry has been immense; it is the gold standard for determination of new drugs structures. A knowledge of drug structure allows identification of the way that the drug interacts with the body. Importantly, crystallography can also be used to demonstrate the purity of prescription drugs: each compound has a unique fingerprint in the way it scatters X-rays – if the pattern contains extra features then the drug has been adulterated. So crystallography has played a pivotal role in the development of safe, effective medicines.
Almost any solid device or appliance has been designed or improved using crystallography in some way. It has shaped our modern world in ways almost too numerous to count.
UNESCO has chosen to celebrate a central science that has helped many discoveries in human life, from the first crystal structure in 1912, the structure of DNA in 1953, to current studies of complex proteins and materials designed to store hydrogen or capture carbon dioxide. But it is not just the Earth where crystallography is used – in September 2013 a team of NASA scientists from led by David Bish of Indiana University reported on the mineralogy of Mars. Their crystallographic experiments carried out on Mars identified the rocks present, and verified the claim about the presence of water on the red planet.
In 2014, science fairs are going to be celebrating crystallography, a quiet unassuming scientific discipline, without which modern life would be very different.
Other articles in this series: Explainer: what is crystallography?
Timothy Prior has received funding from Engineering and Physical Sciences Research Council under grant number EP/I028692/1.
This article was originally published on The Conversation. Read the original article. The views expressed are those of the author and do not necessarily reflect the views of the publisher. This version of the article was originally published on Live Science.