Can Bleach Help Solve the Origin of Life in the Primordial Soup? (Op-Ed)

This article was originally published at The Conversation. The publication contributed the article to Live Science's Expert Voices: Op-Ed & Insights.
A chemical found in hair bleach may help answer questions about the origins of life and explain why new life does not emerge on modern Earth.
Hydrogen peroxide may have helped transform RNA (ribonucleic acid) into one of the building blocks of life, we found in a study published today in Journal of the Royal Society Interface.
More than 3.6 billion years ago there were no living cells and no proteins in the primordial soup on earth.
The RNA world hypothesis holds that cell-free communities grew in rock pores around hydrothermal vents and replicated and evolved, before the evolution of DNA and cell membranes.
But cell-free RNA replication requires thermal cycling – heating to separate the base-paired double strands and a cooling phase to anneal complementary strands into newly replicated double helices.
This fact is often overlooked in hypotheses about the origin of life, although the polymerase chain reaction (PCR) method routinely used to amplify DNA in the lab uses artificially imposed thermal cycling.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
So what mechanism may have provided spontaneous, self-sustained thermal cycling on the ancient earth?

The breakthrough
Our study brought together interdisciplinary researchers at the Australian National University and Leeds University, UK.
Bringing insights gained from applied mathematics and chemical engineering to bear on a problem that has been tackled by chemists and molecular biologists, we described and tested a previously unrecognised mechanism for driving a replicating molecular system on the pre-biotic earth.
Researchers had suggested earlier that thermal cycling may have been provided by convective oscillations in millimetre-sized rock pores.
We proposed that thermal cycling in the primordial soup may have been provided by a natural thermochemical oscillator, driven by spontaneous, exothermic (heat producing) reactions of hydrogen peroxide.
A thermochemical oscillator is an exothermally reacting chemical system that gives a periodic temperature response. They have been studied experimentally since 1969.
Hydrogen peroxide is a simple molecule with the chemical formula H₂O₂. It is made and used in vast quantities in the polymer industry and has some domestic uses in hair bleach and antiseptics, but it also occurs in small quantities naturally on the Earth and in the biosphere.
Oscillatory thermoconversion is typical of highly energetic, thermally sensitive, liquids such as hydrogen peroxide.
Such liquids have high specific heat capacity so their intermolecular bonds can absorb much of the heat of reaction. But when the bonds cannot absorb any more heat the temperature spikes to a maximum, then declines to a minimum, and the cycle begins again.
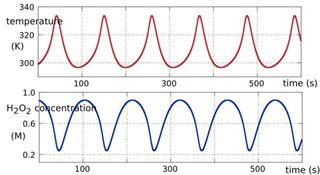
The hydrogen peroxide oscillator turns out to have just the right period – around 90 seconds – to drive the replication of small RNAs. If the period is too long the RNAs decay faster than replication can increase them. If the period is too short the strands do not separate completely and replication fails.
Replication, amplification and evolution
We set up detailed computational simulations and found that the hydrogen peroxide oscillator can indeed drive rapid RNA replication and amplification.
But there’s more. In the presence of RNA template strands the oscillatory system can become quasi-periodic, and the thermal oscillations can take on more complex forms – biperiodic, for example.
This may lend additional, powerful capabilities to a molecular replicating system. A biperiodic temperature response is capable of replicating two different RNA species, and nature may well have done exactly that in the primordial rock pores.
How might complementary RNA strands have been produced in the pre-biotic primordial soup? Well, it has been shown that long polynucleotides can be synthesised on mineral surfaces. We proposed a surface-promoted scheme, which itself may be driven by the hydrogen peroxide oscillator.
A truly living system must evolve, as well as replicate. Now, RNA is not totally stable in the presence of hydrogen peroxide. This is good, because it allows for some infidelity in replication.
In other words, we also have evolution! RNA that is modified by the action of hydrogen peroxide in such a way that confers resilience to hydrogen peroxide damage would, of course, be selected for. We have natural selection, too!
Other worlds
Experiments have indicated that hydrogen peroxide was present on the early Earth, and may easily have occurred in high enough concentrations to undergo oscillatory thermoconversion in hydrothermal rock pores.
Hydrogen peroxide is known also to occur abundantly on Jupiter’s moon Europa, and is believed to have occurred formerly on Mars, which suggests that these planetary bodies may have evolved their own RNA worlds!
Our results also may provide an answer to the (previously unanswerable) question of why life does not emerge from non-living precursors on the modern earth. We do not find spontaneously self-replicating and evolving RNA communities around modern hydrothermal vents.
The answer? Quite simply there are no longer the amounts of hydrogen peroxide in those environments that were there in the good old days!
Rowena Ball receives funding from the Australian Research Council.
This article was originally published on The Conversation. Read the original article. The views expressed are those of the author and do not necessarily reflect the views of the publisher. This version of the article was originally published on Live Science.