History of Chemistry | Famous Chemists
In many ways, the history of civilization is the history of chemistry — the study of matter and its properties. Humans have always sought to identify, use and change the materials in our environment. Early potters found beautiful glazes to decorate and preserve their wares. Herdsmen, brewers and vintners used fermentation techniques to make cheese, beer and wine. Housewives leached the lye from wood ash to make soap. Smiths learned to combine copper and tin to make bronze. Crafters learned to make glass; leatherworkers tanned hides.
In the eighth century A.D., Jābir ibn Hayyān, a Muslim astronomer, philosopher and scientist, became one of the first to use scientific methods to study materials. Also known by his Latinized name, Geber, he is known as the "father of chemistry." He is thought to be the author of 22 scrolls describing methods of distillation, crystallization, sublimation and evaporation. He invented the alembic, a device used to distill and study acids. He also developed an early chemical classification system using the properties of the materials he studied. His categories were:
- “Spirits” — materials that would vaporize when heated.
- "Metals" — including iron, tin, copper, and lead.
- Non-malleable substances — materials that could be made into powders, such as stone.
Today we might call similar materials “volatile chemicals, metals and non-metals.”
Classical chemistry
In Europe, the study of chemistry was conducted by alchemists with the goals of transforming common metals into gold or silver and inventing a chemical elixir that would prolong life. Although these goals were never achieved, there were some important discoveries made in the attempt.
Robert Boyle(1627-1691) studied the behavior of gases and discovered the inverse relationship between volume and pressure of a gas. He also stated that “all reality and change can be described in terms of elementary particles and their motion,” an early understanding of atomic theory. In 1661, he wrote the first chemistry textbook, “The Sceptical Cymist,” which moved the study of substances away from mystical associations with alchemy and toward scientific investigation.
By the 1700s, the Age of Enlightenment had taken root all over Europe. Joseph Priestley (1733-1804) disproved the idea that air was an indivisible element. He showed that it was, instead, a combination of gases when he isolated oxygen and went on to discover seven other discreet gases. Jacques Charlescontinued Boyles’ work and is known for stating the direct relationship between temperature and pressure of gases. In 1794, Joseph Proust studied pure chemical compounds and stated the Law of Definite Proportions — a chemical compound will always have its own characteristic ratio of elemental components. Water, for instance, always has a two-to-one ratio of hydrogen to oxygen.

Antoine Lavoisier (1743-1794) was a French chemist who made important contributions to the science. While working as a tax collector, Lavoisier helped to develop the metric system in order to insure uniform weights and measures. He was admitted to the French Academy of Sciences in 1768. Two years later, at age 28, he married the 13-year-old daughter of a colleague. Marie-Anne Lavoisier is known to have assisted her husband in his scientific studies by translating English papers and doing numerous drawings to illustrate his experiments.
Lavoisier’s insistence on meticulous measurement led to his discovery of the Law of Conservation of Mass. In 1787, Lavoisier published "Methods of Chemical Nomenclature," which included the rules for naming chemical compounds that are still in use today. His "Elementary Treatise of Chemistry" (1789) was the first modern chemistry textbook. It clearly defined a chemical element as a substance that cannot be reduced in weight by a chemical reaction and listed oxygen, iron, carbon, sulfur and nearly 30 other elements then known to exist. The book did have a few errors though; it listed light and heat as elements.
Amedeo Avogadro (1776-1856) was an Italian lawyer who began to study science and mathematics in 1800. Expanding on the work of Boyle and Charles, he clarified the difference between atoms and molecules. He went on to state that equal volumes of gas at the same temperature and pressure have the same number of molecules. The number of molecules in a 1-gram molecular weight (1 mole) sample of a pure substance is called Avogadro’s Constant in his honor. It has been experimentally determined to be 6.023 x 1023 molecules and is an important conversion factor used to determine the mass of reactants and products in chemical reactions.
In 1803, an English meteorologist began to speculate on the phenomenon of water vapor. John Dalton (1766-1844) was aware that water vapor is part of the atmosphere, but experiments showed that water vapor would not form in certain other gases. He speculated that this had something to do with the number of particles present in those gases. Perhaps there was no room in those gases for particles of water vapor to penetrate. There were either more particles in the “heavier” gases or those particles were larger. Using his own data and the Law of Definite Proportions, he determined the relative masses of particles for six of the known elements: hydrogen (the lightest and assigned a mass of 1), oxygen, nitrogen, carbon, sulfur and phosphorous. Dalton explained his findings by stating the principles of the first atomic theory of matter.
- Elements are composed of extremely small particles called atoms.
- Atoms of the same element are identical in size, mass and other properties. Atoms of different elements have different properties.
- Atoms cannot be created, subdivided or destroyed.
- Atoms of different elements combine in simple whole number ratios to form chemical compounds.
- In chemical reactions atoms are combined, separated or rearranged to form new compounds.
Dmitri Mendeleev (1834-1907) was a Russian chemist known for developing the first Periodic Table of the Elements. He listed the 63 known elements and their properties on cards. When he arranged the elements in order of increasing atomic mass, he could group elements with similar properties. With a few exceptions, every seventh element had similar properties (The eighth chemical group — the Noble Gases — had not been discovered yet). Mendeleev realized that if he left spaces for the places where no known element fit into the pattern that it was even more exact. Using the blank spaces in his table, he was able to predict the properties of elements that had yet to be discovered. Mendeleev’s original table has been updated to include the 92 naturally occurring elements and 26 synthesized elements.
Describing the atom
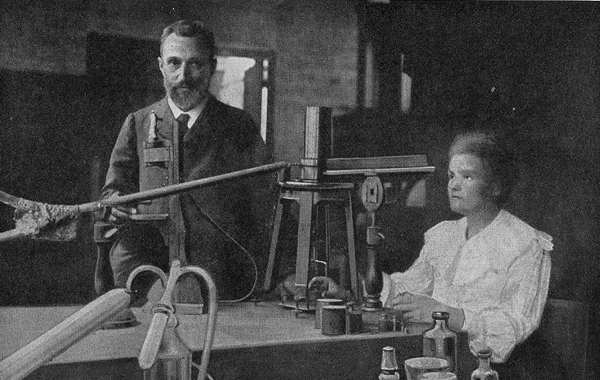
In 1896, Henri Becquerel discovered radiation. Along with Pierre and Marie Curie, he showed that certain elements emit energy at fixed rates. In 1903, Becquerel shared a Nobel Prize with the Curies for the discovery of radioactivity. In 1900, Max Planck discovered that energy must be emitted in discreet units that he called “quanta” (since named photons) not in continuous waves. It appeared that atoms were made up of still smaller particles, some of which could move away.
In 1911, Ernst Rutherford demonstrated that atoms consisted of a tiny dense positively charged region surrounded by relatively large areas of empty space in which still smaller, negatively charged particles (electrons) move. Rutherford assumed that the electrons orbit the nucleus in separate neat orbits, just as the planets orbit the sun. However, because the nucleus is larger and denser than the electrons, he could not explain why the electrons were not simply pulled into the nucleus thus destroying the atom.

Niels Bohr’s (1885-1962) atomic model solved this problem by using Planck’s information. Photons are emitted from an electrically stimulated atom only at certain frequencies. He hypothesized that electrons inhabit distinct energy levels and light is only emitted when an electrically “excited” electron is forced to change energy levels.
Electrons in the first energy level, closest to the nucleus, are tightly bound to the nucleus and have relatively low energy. In levels more distant from the nucleus the electrons have increasing energy. Electrons in the energy level furthest from the nucleus are not bound as tightly and are the electrons involved when atoms bond together to form compounds. The periodic nature of the elemental properties is a result of the number of electrons in the outer energy level that can be involved in chemical bonds. Although Bohr models have been replaced by more accurate atomic models, the underlying principles are sound and Bohr models are still used as simplified diagrams to show chemical bonding.
Our understanding of the atom has continued to be refined. In 1935, James Chadwick was awarded the Nobel Prize for his discovery that there are an equal number of electrically neutral particles in the nucleus of an atom. Since neutrons are electrically neutral, they are not deflected by either electrons or protons. Furthermore, neutrons have more mass than protons. These facts combine to make it possible for neutrons to penetrate atoms and break apart the nucleus, releasing vast amounts of energy. In recent years, it is increasingly obvious that the protons, neutrons and electrons of classical chemistry are made up of still smaller subatomic particles. The sciences of chemistry and physics are becoming increasingly intertwined and theories overlap and conflict as we continue to probe the materials out of which our universe is made.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.