How to Turn an Inkjet Printer into a Bio Lab (Op-Ed)
This article was originally published at The Conversation. The publication contributed the article to Live Science's Expert Voices: Op-Ed & Insights.
If you stop and think about it for a moment, you will realise what an astonishing feat of precision engineering your colour printer is. It can take the primary colours – cyan, yellow, magenta and black – and mix them together carefully enough to achieve more than a million different hues and shades. Not only that but the drops of colour are mere nanolitres (billionths of a litre) in volume, each of which is then placed on the paper – assuming its not jammed in the feeder tray – with better than pinpoint accuracy.
Now a group of enterprising chemists from Tsinghua University are exploiting that precision engineering, which normally results in high-resolution colour prints, to screen millions of different chemical reactions. Their results have been published in the journal Chemical Communications.
Yifei Zhang and colleagues have been trying to understand reaction pathways in living things. Every chemical process that goes on in living organisms is controlled by a cascade of reactions. The steps in a cascade are mediated by protein molecules called enzymes. Each enzyme makes a small chemical alteration, like workers on a production line, to a molecule before passing its product onto the next enzyme. In this way, for example, plants build sugars from carbon dioxide and your food gets broken down and then reconstructed into other useful chemicals for your body.
The problem is that to understand these complicated processes by reconstructing them outside of a living cell is difficult. The concentrations of an enzyme relative to the next in the line is key. Get this wrong and bottle necks are formed in the production line, as one enzyme works faster than the next.
To figure out what are the right conditions to replicate a living cell’s workings, chemists must set up and monitor a vast number of reactions. Screening large numbers of reactions like this is often done using “96-well plates”, which are 96 tiny containers with a unique combination of chemicals in each. These reactions might be set up manually or, if the lab is well-funded, by an expensive robot. But even with the best robots available it can still be a slow process.
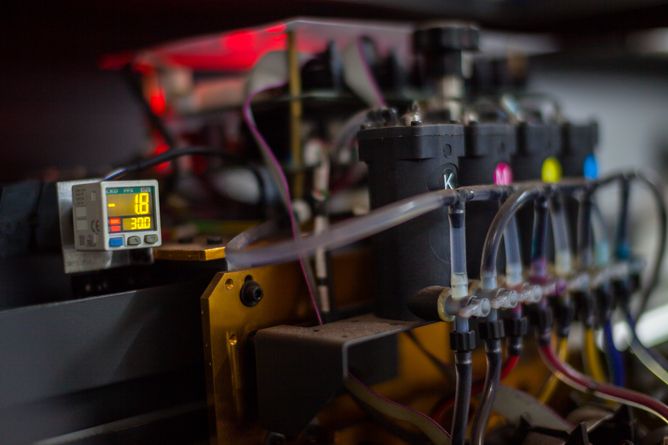
Colour printers are a lot cheaper than robots. And if the inks are replaced by solutions of enzymes then suddenly you have a device that has the potential to dispense more than a million different reaction mixtures.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
That is just want Yifei and colleagues have done. Their printers were loaded with a series of enzymes that, when they work together in the correct ratios, produce coloured reaction products. These were printed directly onto paper where it was immediately obvious, from the intensity of a coloured dot, which reaction mixtures worked best.
In the test cases reactions were deliberately chosen that resulted in colour changes. This made for a nice quick visual indication of whether the system worked well. So for example one test started with glucose and a chemical called ABTS in the magenta cartridge, then the enzymes glucose oxidase (GOx) and horse-radish peroxidase (HRP) in the yellow and cyan cartridges. When they are mixed together the GOx removes a hydrogen from the glucose and adds it to oxygen, producing hydrogen peroxide. Next the HRP reacts this with the ABTS, which results in a green chemical.
The potential applications for these printer-based mixtures extend beyond curiosity-driven research on biological pathways. Yifei and colleagues have already shown that by loading the printer cartridges with the right enzymes they can use the set up to indicate the presence of glucose in a sample. Glucose in urine is a indication of diabetes, so their printer-based chemistry already has the potential to diagnose diabetes.
The result then could be a future where a trip to the doctors results in a printout of, quite literally, your urine and some enzymes alongside, after 30 seconds or so, a diagnosis and the prescription.
Mark Lorch does not work for, consult to, own shares in or receive funding from any company or organisation that would benefit from this article, and has no relevant affiliations.
This article was originally published on The Conversation. Read the original article. Follow all of the Expert Voices issues and debates — and become part of the discussion — on Facebook, Twitter and Google +. The views expressed are those of the author and do not necessarily reflect the views of the publisher. This version of the article was originally published on Live Science.

Mark Lorch is Professor of Science Communication at the University of Hull. He trained as a protein chemist, studying protein folding and function. His research now focuses on the chemistry of a broad range of biological systems including lipids, proteins and even plant spores. As well as his written outputs he gives regular talks to schools, the public and conferences, and he occasionally pops up on the radio and TV explaining science and technology to a public audience.