Melanoma Tumor 'Dissolves' After 1 Dose of New Drug Combo
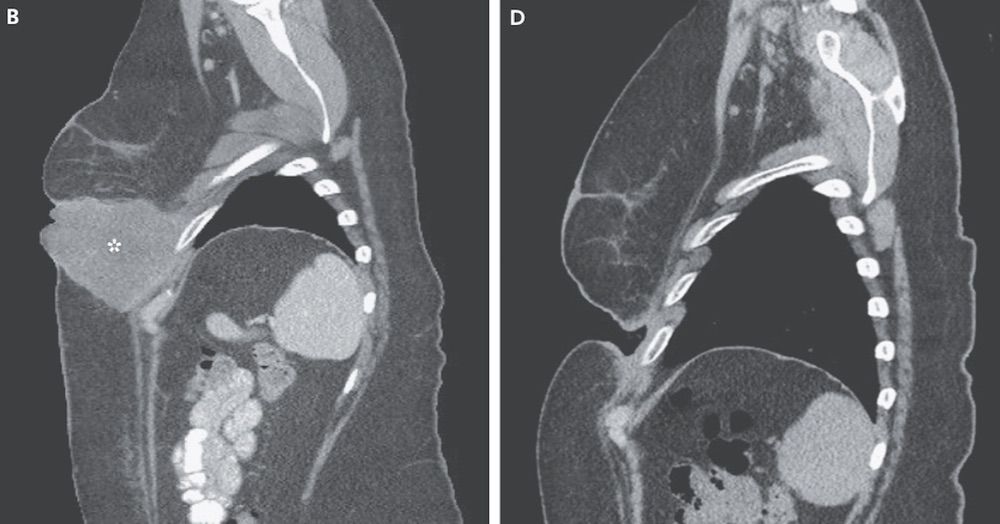
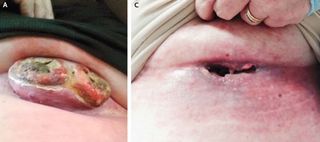
A large melanoma tumor on a woman's chest disappeared so quickly that it left a gaping hole in its place after she received a new treatment containing two melanoma drugs, a new case report finds.
Doctors are still monitoring the 49-year-old woman, but she was free of melanoma — a type of skin cancer that can be deadly — at her last checkup, said the report's lead author, Dr. Paul Chapman, an attending physician and head of the melanoma section at the Memorial Sloan Kettering Cancer Center in New York.
The woman took the same two drugs as more than 100 people with melanoma who took part in a recent study. For most of the study participants who took these drugs, the combination worked better than one drug alone. But the doctors were surprised by how well the drug combination worked to treat this particular woman's cancer — they had not anticipated that a melanoma tumor could disappear so quickly that it would leave a cavity in the body — and thus wrote the report describing her case.
"What was unusual was the magnitude [of recovery], and how quickly it happened," Chapman told Live Science. However, doctors are wary of the drug combination because it does not work for everyone, and can have side effects, such as severe diarrhea. [10 Do's and Don'ts to Reduce Your Risk of Cancer]
Both the study of the drug combination and the woman's case report were published Monday (April 20) in the New England Journal of Medicine. The drug combination is part of a relatively recent approach to treating melanoma with medications that boost a person's own immune system, called immunotherapy.
One of the drugs in the combination was ipilimumab (sold under the brand name Yervoy), which works by removing an inhibitory mechanism that can stop certain immune cells from killing cancer cells.
In the study, researchers combined ipilimumab with another drug, called nivolumab (brand name Opdivo), which can prevent immune cells called T cells from dying, Chapman said.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The U.S. Food and Drug Administration has approved ipilimumab and nivolumab separately as melanoma drugs but has not approved their combined use. The researchers' study was aimed at testing how the two drugs worked when used in tandem.
In the study, doctors gave treatments to 142 people with metastatic melanoma (melanoma that has spread to other parts of the body) — some participants received the combination, and others received ipilimumab plus a placebo. Neither the participants nor their doctors knew who had received which treatment until the trial had ended.
The new drug combination had better results than the ipilimumab-plus-placebo treatment, the researchers found.
In one analysis, the researchers focused on 109 patients who did not have a mutation in a gene called the BRAF gene. (BRAF mutations are linked to a number of cancers, including melanoma, and there are other melanoma drugs that target BRAF mutations.) Among the 72 people in this group who took the combination, 61 percent saw their cancer shrink, compared with just 11 percent of the 37 people in the group who took only ipilimumab.
What's more, melanoma was undetectable in 22 percent of the combination group at the end of the study, which was funded by Bristol-Myers Squibb, which makes the drugs. None of the people taking ipilimumab plus a placebo saw their melanoma disappear by the time the study had ended.
Twenty-two percent may not sound high, but in the world of melanoma treatment, it is significant, said Dr. Sylvia Lee, an assistant professor of medicine at the University of Washington, Seattle Cancer Care Alliance and Fred Hutchinson Cancer Research Center. Lee was not involved in the new study, but she is working with patients who are receiving the drug combination in Seattle.
A complete response to treatment is "the Holy Grail," she said. "That's what everyone wants, where all of the cancer disappears. We're talking about patients with stage IV melanoma. Usually, in cancers, when someone has stage IV disease, for the majority of people, it's no longer curable." [Medicine's Journey Through the Body: 4 Stages]
It's unclear whether melanoma will reoccur in any of the patients in the new study. Doctors are following them to see whether the people who are taking the combination drugs live longer than expected, Chapman said.
Side effects
However, the ipilimumab with nivolumab combination comes with serious side effects, such as colitis (swelling of the colon), diarrhea and problems with the endocrine glands (which produce hormones).
About 54 percent of the patients in the study who were taking the combination reported serious side effects, compared with 24 percent of the people taking only ipilimumab, the researchers found.
The treatments are given three weeks apart, but some people can tolerate only one or two treatments out of the suggested four before they stop taking the medicine, Lee said. In the new study, about 60 percent of the participants taking the combination finished all four treatments, compared with 70 percent of the ipilimumab-only group.
The side effects can be brutal, Lee said. "This is diarrhea that is 25 to 40 times a day," she said.
Future trials may help researchers refine the number of treatments needed and figure out how effective just one or two treatments can be. The current trial is over, but certain cancer centers are still offering the drug combination through an expanded access program, which is how the woman whose tumor disappeared got the medicine.
Her case shows that immunotherapy can work quickly: Her tumor vanished within three weeks of receiving her first treatment, the researchers found.
"I was astonished; I'd never seen anything like that," Chapman said. "She said the tumor had just kind of dissolved."
However, the combination may pose a risk if it dissolves a tumor somewhere else the body, and leaves a hole behind.
"I think that it is a huge concern," Lee said. "It is something to consider if you do have a patient with a tumor [invading] a vital organ."
The medications are also pricey. Ipilimumab costs $120,000 for four treatments, and nivolumab is priced at $12,500 a month, the Wall Street Journal reported.
Still, the drug combination may offer a new and promising treatment for people with melanoma if the FDA approves it, Chapman said.
"It kind of confirms an assumption that we've all had for many decades: that the immune system can recognize cancers and can kill large tumors if properly activated," Chapman said.
Follow Laura Geggel on Twitter @LauraGeggel. Follow Live Science @livescience, Facebook & Google+. Original article on Live Science.

Laura is the archaeology and Life's Little Mysteries editor at Live Science. She also reports on general science, including paleontology. Her work has appeared in The New York Times, Scholastic, Popular Science and Spectrum, a site on autism research. She has won multiple awards from the Society of Professional Journalists and the Washington Newspaper Publishers Association for her reporting at a weekly newspaper near Seattle. Laura holds a bachelor's degree in English literature and psychology from Washington University in St. Louis and a master's degree in science writing from NYU.