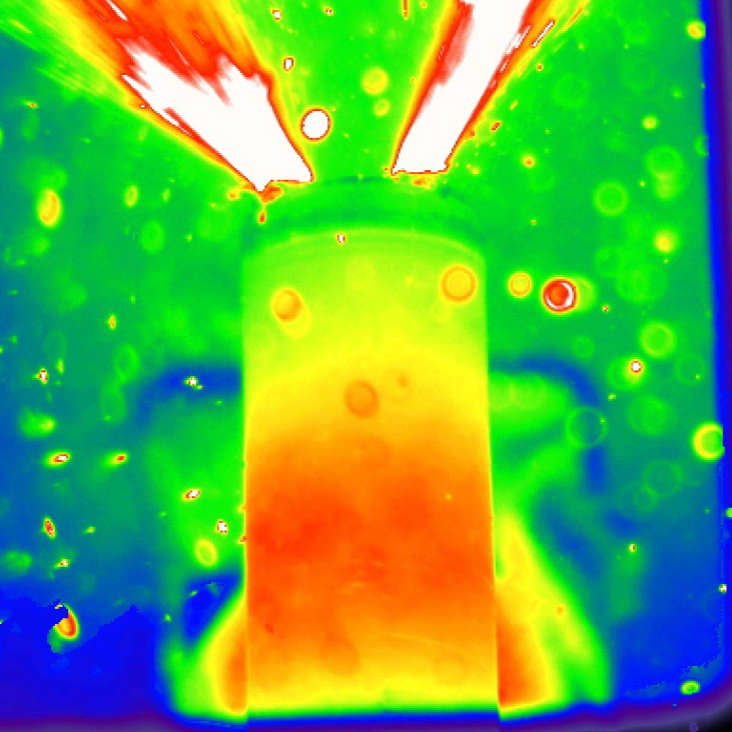
Real-time images have captured the chain reaction that causes lithium-ion batteries to explode. .
The process can occur in just milliseconds: Overheated battery modules create a domino effect, producing more and more heat, and the battery explodes. But it turns out that not all batteries are equally likely to fail, according to a new study published today (April 28) in the journal Nature Communications.
"The presence of certain safety features can mitigate against the spread of some of this thermal runaway process," said study co-author Paul Shearing, a chemical engineer at the University College London in the United Kingdom. Those features include mechanical supports inside the battery, Shearing said.
The results suggest some ways to make rechargeable lithium-ion batteries safer, the researchers wrote in the paper. [9 Odd Ways Your Tech Device May Injure You]
Rechargeable batteries
Lithium-ion batteries are the workhorses of modern-day gadgets; they're found in everything from smartphones to jumbo jets to the Tesla Model S. They are typically made with two layers of material, called the anode and the cathode, separated by an electrically conducting fluid. Lithium ions start off in the cathode, a layer of material that, in laptop and cellphone batteries, typically includes cobalt, manganese, nickel and oxygen. When the batteries are charged, electricity drives the lithium ions from the cathode, across an ion-filled electrolyte fluid, and into the anode, which is made of stacks of graphite. As the battery drains, the lithium ions return from the anode back into the cathode. The batteries typically come in cells; a laptop battery may have three or four cells, whereas a Tesla Model S may have thousands, Shearing said.
Chain reaction
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Hundreds of millions of lithium-ion batteries are produced every year, and catastrophic failure, such as explosion or melting, is rare, Shearing said. Still, there have been 43 product recalls for defective lithium-ion batteries since 2002, according to the U.S. Consumer Product Safety Commission.
Batteries can blow up or melt when internal electrical components short-circuit, when mechanical problems crop up after a fall or an accident, or when they are installed incorrectly, Shearing said. But at the heart, all of these failures occur because one portion of the battery gets too hot and can't cool down quickly enough, creating a chain reaction that generates more and more heat.
"It's kind of this snowball process that we call thermal runaway," Shearing told Live Science.
During thermal runaway, the miniature battery modules can melt, giving off heat, and the electrolyte material between the anode and the cathode may even boil, Shearing said.
![Typical batteries are powered by a chemical reaction. [See full infographic]](https://cdn.mos.cms.futurecdn.net/ua6pz8XQurFaYvJrZxJGAH.jpg)
To understand more about this dangerous chain reaction, Shearing and his colleagues heated commercial lithium-ion batteries to 482 degrees Fahrenheit (250 degrees Celsius). Using a high-speed 3D camera and a particle collider, which bombarded the batteries with synchrotron X-rays, the team captured thermal images of the batteries as they underwent the flash transition to overheating and thermal runaway.
Safer batteries
Even at high temperatures, not all of the batteries failed — some had internal safety features that prevented the dangerous reaction. Of those that did fail, the batteries with internal supports stayed intact until the internal temperature reached a scorching 1,830 F (1,000 C). At that point, the internal copper materials melted, leading to the runaway chain reaction.
But the batteries without these internal supports exploded, likely because their internal cores collapsed, which could have short-circuited the internal electrical components, the study showed.
The new technique provides a way to systematically test safety features in batteries in the future, Shearing said.
Even though exploding batteries sound frightening, they're actually quite rare, Shearing said. After all, most people don't bake their iPhones during daily use, he said.
"We had to push these into really extreme conditions, which [you] are very unlikely to see in your normal day-to-day operations," Shearing said.
Follow Tia Ghose on Twitter and Google+. Follow Live Science @livescience, Facebook & Google+. Originally published on Live Science.

Tia is the managing editor and was previously a senior writer for Live Science. Her work has appeared in Scientific American, Wired.com and other outlets. She holds a master's degree in bioengineering from the University of Washington, a graduate certificate in science writing from UC Santa Cruz and a bachelor's degree in mechanical engineering from the University of Texas at Austin. Tia was part of a team at the Milwaukee Journal Sentinel that published the Empty Cradles series on preterm births, which won multiple awards, including the 2012 Casey Medal for Meritorious Journalism.









