Liquid water flows on Mars today, boosting the odds that life could exist on the Red Planet, a new study suggests.
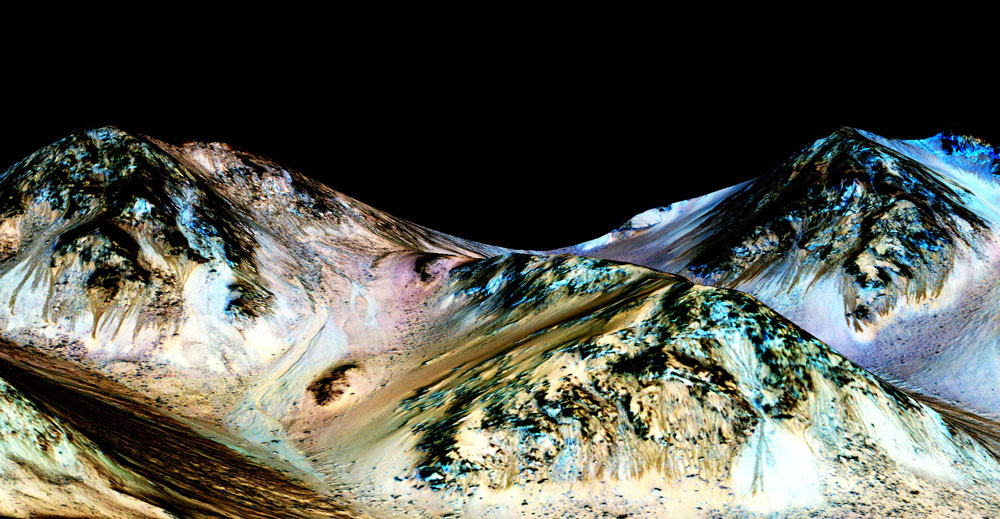
The enigmatic dark streaks on Mars — called recurring slope lineae (RSL) — that appear seasonally on steep, relatively warm Martian slopes are likely caused by salty liquid water, researchers said.
"Liquid water is a key requirement for life on Earth," study lead author Lujendra Ojha, of the Georgia Institute of Technology in Atlanta, told Space.com via email. "The presence of liquid water on Mars' present-day surface therefore points to environment[s] that are more habitable than previously thought." [Flowing Water on Mars: The Discovery in Pictures ]
Ojha was part of the team that first discovered RSL in 2011, by studying images captured by the High Resolution Imaging Science Experiment (HiRISE) camera aboard NASA's Mars Reconnaissance Orbiter (MRO).
RSL occur in many different locations on Mars, from equatorial regions up to the planet's middle latitudes. These streaks are just 1.6 feet to 16 feet (0.5 to 5 meters) wide, but they can extend for hundreds of meters downslope.
RSL appear during warm weather but fade away when temperatures drop, leading many researchers to speculate that liquid water is involved in their formation. The new study, which was published online today (Sept. 28) in the journal Nature Geoscience, strongly supports that hypothesis, team members said.
Ojha and his colleagues scrutinized data gathered about four different RSL locations by another MRO instrument, the Compact Reconnaissance Imaging Spectrometer for Mars (CRISM).
Get the world’s most fascinating discoveries delivered straight to your inbox.
"Using this instrument, we can deduce the mineralogical makeup of surface materials on Mars," Ojha said. "What we found was that at times and places when we see biggest RSL on the surface of Mars, we also found spectral evidence for hydrated salts on the slopes where RSL form."
Hydrated salts precipitate from liquid water, so detecting them is a big deal — especially since circumstances make it unlikely that CRISM could spot RSL water directly. (CRISM observes the Red Planet at the driest time of the Martian day, about 3 p.m., when any liquid surface water would likely have evaporated, Ojha said.)
No planet is more steeped in myth and misconception than Mars. This quiz will reveal how much you really know about some of the goofiest claims about the red planet.
Mars Myths & Misconceptions: Quiz
"Due to that, I do not think we will ever find the RSL still in their liquid form at 3:00 p.m., so I think this hydrated signature of the salts is definitely a 'smoking gun,'" he said.
The RSL-associated salts appear to be perchlorates, a class of chlorine-containing substances that are widespread on Mars. These salts lower the freezing point of water from 32 degrees Fahrenheit (0 degrees Celsius) to minus 94 F (minus 70 C), Ojha said.
"This property vastly increases the stability of brine [salty water] on Mars," he said.
Perchlorates can absorb atmospheric water, Ojha said. But it's unclear if Mars' air is the source of the brine flows. Other possibilities include melting of surface or near-surface ice or discharges of local aquifers.
"It is conceivable that RSL are forming in different parts of Mars through different formation mechanisms," the study team writes in the new paper.
Observations by NASA's Curiosity rover and other spacecraft have shown that, billions of years ago, the Red Planet was a relatively warm and wet world that could have supported microbial life, at least in some regions.
Mars is extremely cold and dry today, which is why the discovery of RSL sites has generated so much excitement over the past four years: The features point to the possibility that simple life-forms could exist on the planet's surface now.
But the new results don't imply that life thrives on Mars today, or even that this is a likely proposition, Ojha stressed. Perchlorate brines have a very low "water activity," he said, meaning that the water within them is not easily available for potential use by organisms.
"If RSL are perchlorate-saturated brines, then life as we know [it] on Earth could not survive in such low water activity," Ojha said.
Follow Mike Wall on Twitter @michaeldwall and Google+. Follow us @Spacedotcom, Facebook or Google+. Originally published on Space.com.