Strange Microbe Lacks Cell's 'Powerhouse'
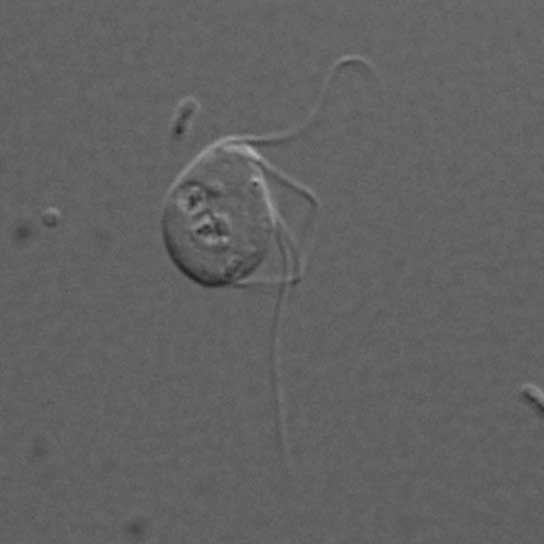
A microbe that lives in the guts of chinchilla is missing mitochondria, the energy-generating cell organelle once thought crucial to the function of eukaryotes.
Eukaryotes are cells with membrane-bound organelles, including a nucleus, a feature that makes them different from prokaryotes (which include bacteria and archaea). One of these membrane-bound organelles is the mitochondria. Mitochondria are known as the cell's "powerhouse" because they create adenosine triphosphate (ATP), which cells use for fuel. Mitochondria are also involved in many other aspects of cell function, said Anna Karnkowska, a co-author on the new study.
In that study, Karnkowska and her colleagues found that the gut-dwelling eukaryote Monocercomonoides does not to have any mitochondria at all. This isn't entirely surprising, on one hand: Many eukaryotes that live in low-oxygen environments have shed their mitochondria because they fuel themselves anaerobically, or without oxygen. (ATP synthesis inside mitochondria requires oxygen.) But all of those organisms have some mitochondria remnants left behind, Karnkowska said. In contrast, Monocercomonoides has nothing. No mitochondria-related proteins. No genes. No related enzymes. [The 12 Weirdest Animal Discoveries]
"It was surprising for us in this context, because we had really lost hope that it could happen," Karnkowska told Live Science.
Iron and sulfur
To fuel its everyday life and growth, Monocercomonoides uses standard anaerobic respiration, Karnkowska said. But the microbe has also replaced many other functions of mitochondria. One of these functions is the assembly of iron-sulfur clusters, one of the most important mitochondrial products used in many reactions around the cell. Iron-sulfur, or Fe-S, clusters can even help regulate which genes are expressed into proteins and which aren't.
Monocercomonoides doesn't have the mitochondrial machinery to make these clusters, but it still assembles them. That's because at some point during its evolution, the microbe acquired some genes from a bacterium in what's called horizontal gene transfer. These genes allow it to mobilize iron and sulfur in the cytosol outside of cell organelles rather than inside mitochondria. Only two other eukaryote lineages are known to have lost their mitochondrial Fe-S machinery. Those microbes also replaced the mitochondrial function with genetic capabilities snatched from bacteria.
"It seems like the last step which has to happen [to lose mitochondria altogether in Monocercomonoides] was this functional replacement of Fe-S cluster machinery" of the cell, Karnkowska said.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Diversity of life
The finding doesn't necessarily downplay the importance of mitochondria to eukaryotic cells, Karnkowska said. Rather, it shows how difficult it is to replace the busy little organelles.
"It's showing which functions are very important for mitochondria and for what reasons, most of the time, mitochondria has to be in the cell," she said. "It's rather like the exception that proves the rule."
But the finding also expands the diversity of the eukaryotes and highlights how little is known about this group. Much more is known about bacteria, which are prokaryotic, because their genomes are smaller and easier to sequence, Karnkowska said. Many single-celled eukaryotes that have been studied are human parasites, such as Giardia intestinalis, because they impact human health. Far less is known about the many eukaryotes living peacefully in marine environments or coexisting in animal digestive tracts, Karnkowska said.
"Microbial eukaryotes, also called protists, are all over everything, inside us, inside other animals, in water, in soil, everywhere," Karnkowska said. "And there's still a lot we just don't know."
Follow Stephanie Pappas on Twitter and Google+. Follow us @livescience, Facebook & Google+. Original article on Live Science.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.
Most Popular