How a Student Photographed a Single Atom With a Store-Bought Camera
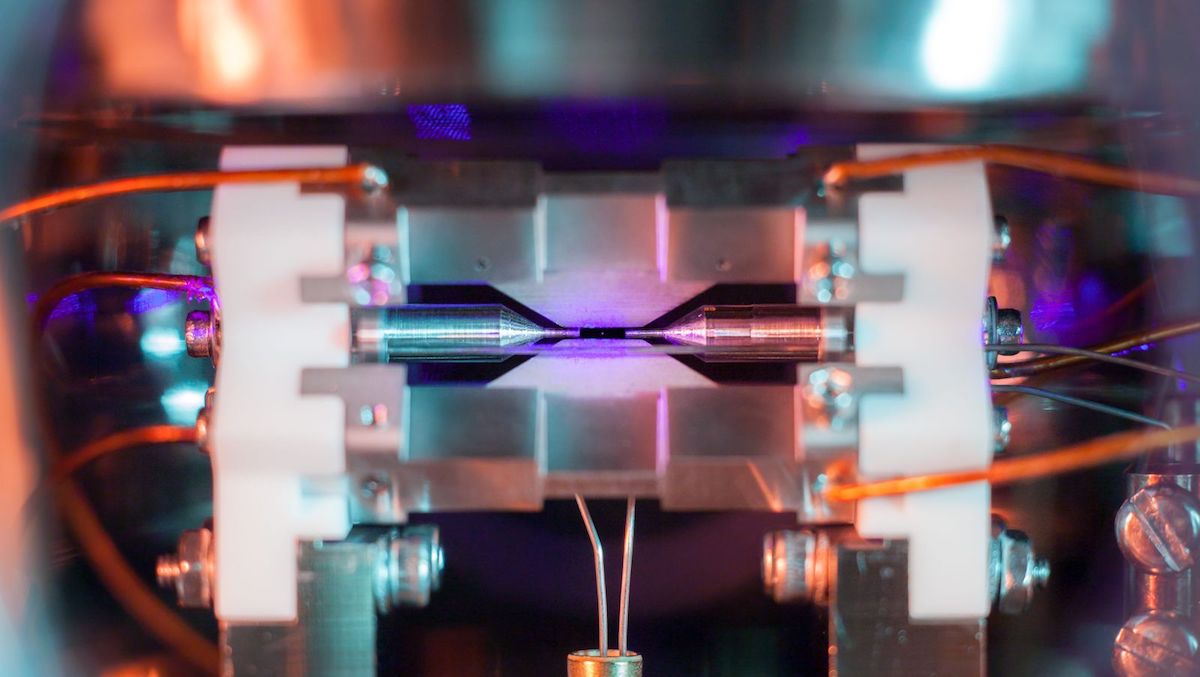
Look closely and you'll see it: a pale, purple pixel hanging in a black field between two cylindrical needles.What looks like a shimmering speck of dust is actually something much, much smaller: a single atom of strontium, isolated in an ion-trap machine at the University of Oxford.
That's small. Really small. Each atom is roughly 0.25 nanometers (or billionths of a meter) across; billions of the atoms would fit comfortably inside a single red blood cell.
How do you capture a photo of something this seemingly infinitesimally small? One photographer, David Nadlinger, used a standard digital camera — but he had some help setting up the shot courtesy of Oxford's Ion Trap Quantum Computing lab, where he is researching for his Ph.D. On Feb. 12, Nadlinger won first place in a national science photography competition organized by the Engineering and Physical Sciences Research Council for capturing this rare photo of a single illuminated atom.
"I think what makes this picture particularly interesting to people is that you can see the surrounding apparatus," Nadlinger told Live Science. "And I think people are also surprised by how big the atom looks here. … I hope I'm not undoing 100 years of science education with this photo — atoms actually are unbelievably small!"
To be clear, Nadlinger said, the purple speck at the center of this photo is not the true size of the strontium atom itself; it's the light from an array of surrounding lasers being re-emitted by the atom. When bathed in a specific wavelength of blue light, strontium creates a glow hundreds of times wider than the radius of the atom itself (which is about a quarter of a nanometer, or 2.5x10 to the -7 meters, Nadlinger said). This glow would be barely perceptible with the naked eye but becomes apparent with a little camera manipulation.
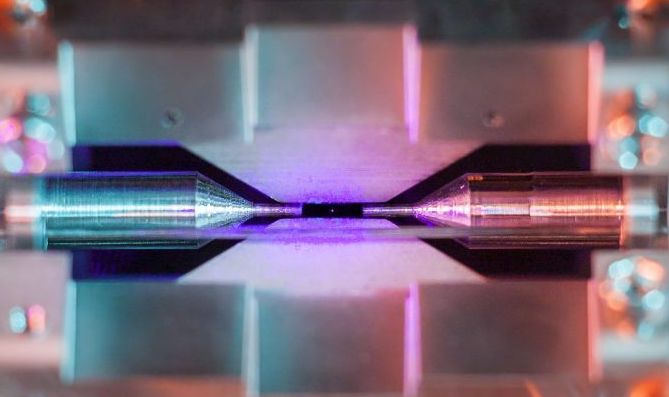
"The apparent size you see in the picture is what we'd call optical aberration," Nadlinger said. "The lens we're seeing it through is not perfect — also it's slightly out of focus and slightly overexposed. You could compare it to looking at the stars in the night sky, which appear bright but are actually much, much smaller than the size they seem to be, just because our eyes (or the camera) don't have enough resolution to process them."
So, seeing a single atom with the naked eye is impossible. Trapping one in a lab, however, is a little more doable.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
To catch an ion by the toe
To make a single atom camera-ready like this, researchers first need to turn it into an ion: an atom with an unequal number of protons and electrons, giving it a positive or negative net charge. "We can only ever trap charged particles," Nadlinger said. "So, we take a stream of neutral strontium atoms, which come from an oven, and shine lasers at them to selectively photo-ionize them. This way, we can create single ions."
When placed in an ion-trap apparatus, single atoms are held in place by four blade-shaped electrodes like those seen above and below the strontium speck in Nadlinger's photo (two additional electrodes are out of view). These electrodes create a current that keeps the atom fixed on the vertical axis; the two needle-shaped cylinders on either side of the atom keep it trapped horizontally.
As the currents from these electrodes interact, they create what is called a rotating saddle potential. "You can see videos online where people literally take a saddle and rotate it and put a ball on it; because of the rotation, the ball actually stays in the center of the saddle. So that's what these electrodes do to confine the ion," Nadlinger said.
Once an atom is confined, an array of lasers hits the atom, which scatters light in all directions; in Nadlinger's photo, you can see traces of the blue laser throughout the background. Using this system, researchers can potentially trap strings of hundreds of ions between the little electrodes, resulting in some stunning imagery.
"On our website, we have a picture of nine ions trapped in a string," Nadlinger said. "In terms of the science, that's actually more interesting than having a single bright pixel surrounded by the ion trap. But to illustrate the concept, this might be more appealing."
Nadlinger does not believe he is the first researcher to take such a photo, but he may well be the most successful at capturing the public's attention with one.
"A group led by Hans Dehmelt, a pioneer of ion trapping and a Nobel laureate [in 1989], once took a picture of a single barium atom in their lab," Nadlinger said. "It was a single bright speck on a dark background, apart from some laser scatter. There's this story that they submitted this image to some conference proceedings — and the image editor just stamped out the ion because he thought it was a speck of dust."
Originally published on Live Science.

Brandon is the space/physics editor at Live Science. His writing has appeared in The Washington Post, Reader's Digest, CBS.com, the Richard Dawkins Foundation website and other outlets. He holds a bachelor's degree in creative writing from the University of Arizona, with minors in journalism and media arts. He enjoys writing most about space, geoscience and the mysteries of the universe.









