These Gut Bugs Need Their Own Gut Bugs
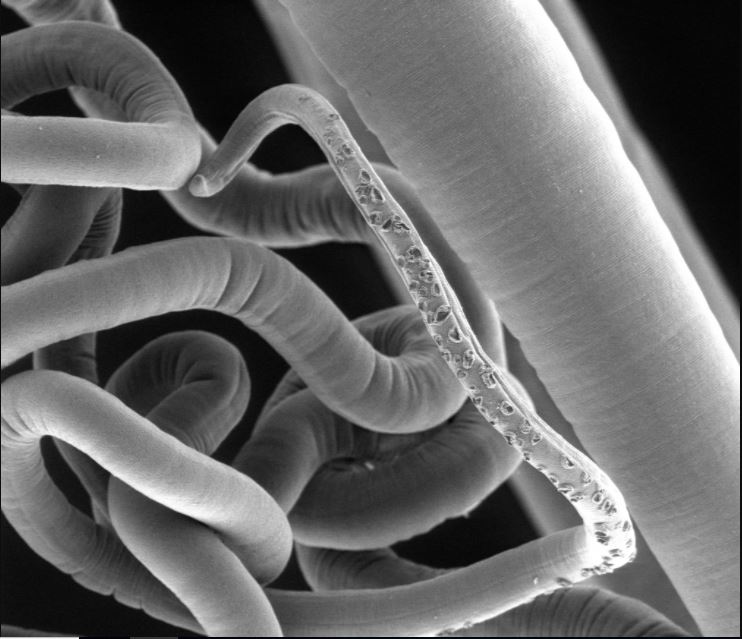
It's a Russian nesting doll of sorts: Parasitic bugs that live in the human gut have their own set of gut bugs inside their intestines.
That's the surprising finding of a new study that examined whipworms (Trichuris trichiura) — parasites that affect an estimated 1 billion people worldwide and can cause diarrhea, vomiting and weight loss, as well as delayed growth in children.
"We were amazed to find that whipworms have their own distinct microflora" and — similar to humans — that the bacteria appear to aid in the parasite's health, study co-author Ian Roberts, a professor of microbiology at the University of Manchester in the United Kingdom, said in a statement. "This shines a light on the fascinating relationships between the parasites, the host and their intestinal dwelling bacteria," Roberts said.
The bacteria inside the parasite's intestine appear to be necessary for its growth, study co-author Richard Grencis, also a professor at the University of Manchester, said in the same statement. What's more, whipworms appear to be able to alter the gut bacteria of their human hosts to aid in their own survival. [8 Awful Parasite Infections That Will Make Your Skin Crawl]
The researchers hope their findings may help lead to the development of more effective drugs for whipworms, which can be difficult to treat.
Whipworm infections occur most frequently in tropical areas with poor sanitation, according to the Centers for Disease Control and Prevention (CDC). The infection spreads when worm eggs get into the soil (through feces), and people touch the contaminated soil and put their hands in their mouths, or they eat fruits or vegetables grown in contaminated soil, the CDC said.
Previous research has focused on how whipworms affect humans and human gut bacteria, but not on the bacteria inside the parasites themselves.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
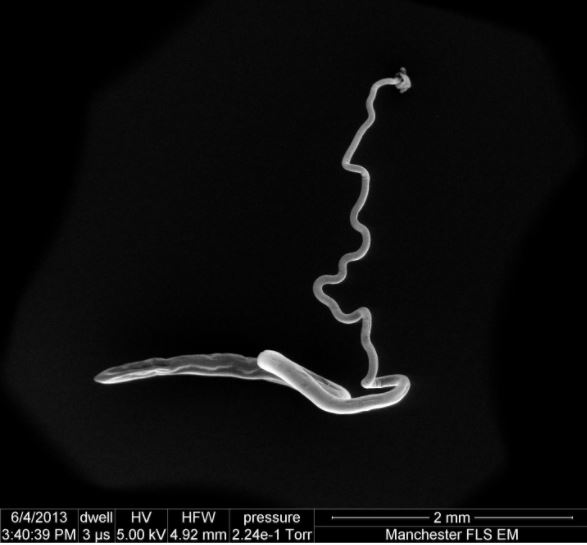
Bacteria in whipworms in mice
In the new study, published today (March 14) in the journal Science Advances, the researchers first took samples of whipworms from infected mice. They found that inside the parasites, there were bacteria, which the parasite acquired from its host. (In this case, the parasites acquired the bacteria from the mouse's gut.) If the parasites were hatched in a bacteria-free environment, they didn't have any gut bacteria.
What's more, the parasites needed this gut bacteria to grow and thrive, the researchers said. When the researchers exposed adult whipworms to antibiotics (which have effects on bacteria rather than parasites), the worms died. But when the researchers exposed young whipworms that were free of bacteria to antibiotics, the drugs didn't have an effect, the researchers said.
In another experiment, the researchers looked at mice that didn't have any gut bacteria (called germ-free mice), and infected the mice with sterile whipworm larvae (whipworm larvae with no bacteria). Two weeks later, these mice had "barely detectable" levels of worms, while mice with normal gut bacteria had high levels of worms.
Interestingly, the researchers found that the composition of gut bacteria inside the adult whipworms was quite different from that of its host. This finding suggests that the whipworm "selects and maintains its own distinct microbiota regardless of the surrounding bacterial populations," the researchers said.
The researchers also found that, once a whipworm infection is established inside a host, the infection results in changes to the host's gut bacteria. This altered gut microbiome reduces the number of new whipworm eggs that can hatch. While this may seem counterproductive for the worm, it keeps amount of the worms from getting too high, and prevents the host's immune system from removing the worms, the researchers said.
The researchers plan to conduct more studies to better understand the role of the whipworm gut bacteria, and how the parasite affects the host microbiome.
Original article on Live Science.

Rachael is a Live Science contributor, and was a former channel editor and senior writer for Live Science between 2010 and 2022. She has a master's degree in journalism from New York University's Science, Health and Environmental Reporting Program. She also holds a B.S. in molecular biology and an M.S. in biology from the University of California, San Diego. Her work has appeared in Scienceline, The Washington Post and Scientific American.










