What's in a Fat Cell?
Fat is so often seen as the enemy — something to avoid or lose. But fat is also a crucial component of the body. Without it, humans would freeze. Our nerves, uninsulated, would jangle with crisscrossed communications. We'd be unable to store crucial supplies of certain vitamins, or have a functioning immune system. On a cellular level, fats make the membranes that surround cells possible and act as messengers that bind to proteins and enable various reactions.
With that in mind, the humble fat cell seems a bit miraculous. Adipocytes, as they're properly known, are the cells that store excess lipids, the molecules that include fats and related substances.
Adipocytes were once thought to be rather dull sacks of energy, but the past few decades of research have revealed that they have a lot to do in the body, from regulating nutrients to releasing hormones that influence blood pressure, thyroid function and even reproduction. [What Is Cellulite?]
Anatomy of fat
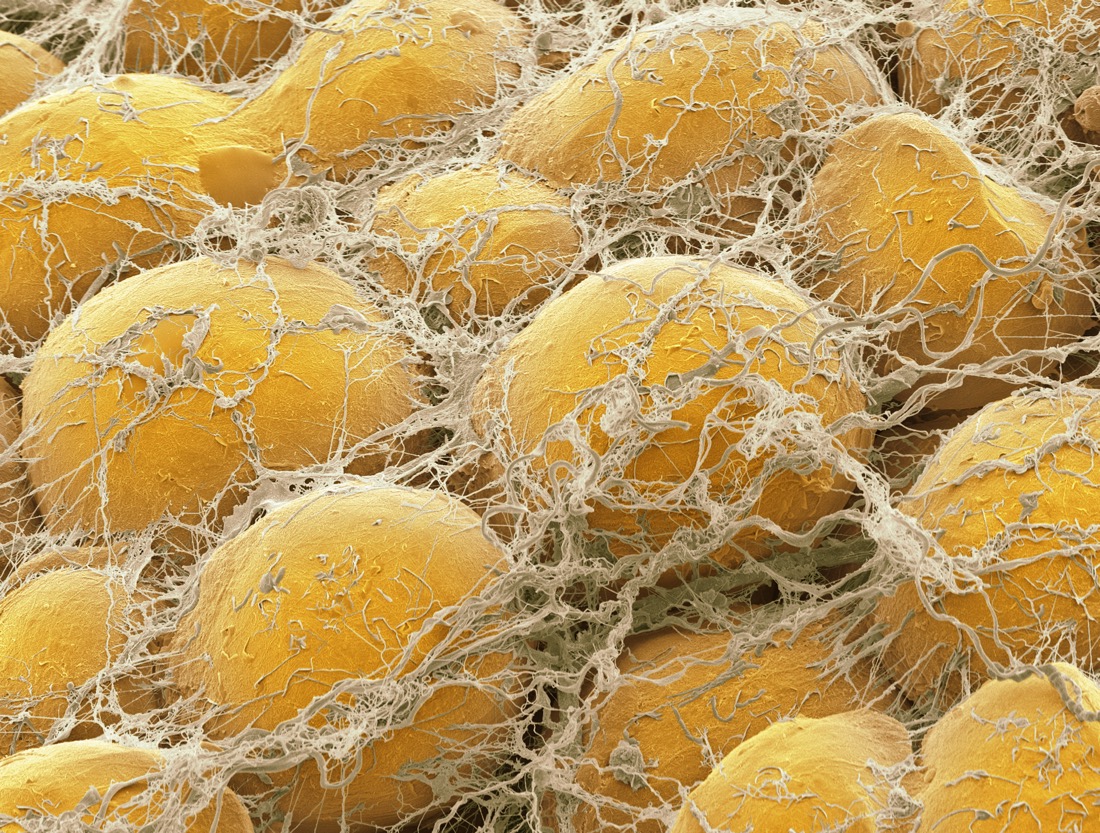
Under a microscope, fat cells look like bulbous little spheres. Like other cells in the body, each has a cell membrane and a nucleus, but their bulk is made up of droplets of stored triglycerides, each of which consists of three fatty-acid molecules attached to a single glycerol molecule.
"Human triglyceride looks exactly like olive oil, peanut oil and all the other triglycerides we squeeze out of plant seeds," said Ruben Meerman, a physicist, science communicator and author of "Big Fat Myths: When You Lose Weight, Where Does the Fat Go?" (Ebury Australia, 2016). "It has the same yellowish color, the same energy density and the exact same chemical formula."
But not all adipocytes are the same. The stuff we typically think of as fat is "white fat," which is the main substance used for energy storage. When insulin levels go up — say, after a meal — white adipocytes take in more fatty acids, literally swelling in size, Meerman told Live Science. When insulin drops, fat cells release their stores as a source of quick energy for the body.
Other clusters of adipocytes are used mostly for support, such as the cushion of fat that surrounds the eyes, according to a 2006 paper in the journal Nature. These fat cells probably don't release a lot of energy into the body unless the organism enters starvation mode. The body also stores fat under the skin (subcutaneous fat) and around the internal organs (visceral fat).
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"Brown fat" cells, on the other hand, are iron-rich cells with their own unique function. They express genes that alter metabolism to produce heat, making brown adipose tissue pretty important for maintaining body temperature. Specifically, brown-fat cells release something called uncoupling protein-1 (UCP-1), which makes the process of fatty-acid oxidation in the cells' powerhouses (the mitochondria) less efficient. That means more of the energy the mitochondria process is "wasted" as heat, thus warming the body, according to a 2017 paper in the journal Endocrine Connections.
Newborn babies have high levels of brown fat. Those levels drop with age, and in adults, most brown fat clusters around the neck and collarbone.
A third type of fat, "beige fat," is found in white adipose tissue, but unlike white-fat cells, these cells contain UCP-1. Beige-fat cells seem to have the flexibility to act like either white fat or brown fat, depending on the situation, according to the Endocrine Connections paper.
What fat can do
Obesity researchers dream of finding ways to turn white fat into energy-burning brown fat. But white fat is pretty neat stuff, too.
Beyond playing a role in providing energy storage, white adipocytes help regulate blood sugar levels. They take up sugar, or glucose, in response to insulin secreted by the pancreas, pulling excess sugar out of the bloodstream. That's one of the big problems with excess body fat, according to the 2006 Nature paper: Too much fat throws off the glucose-regulating function of adipocytes (as does too little fat), and blood sugar levels can be thrown out of whack. [Can You Turn Fat Into Muscle?]
Adipocytes also secrete multiple proteins that influence blood sugar, according to the same paper. Some — such as leptin, adiponectin and visfatin — decrease the levels of glucose in the bloodstream. Others, such as resistin and retinol-binding protein 4, increase blood sugar.
Fatty tissue also plays a role in the immune system. Adipocytes release inflammatory compounds called cytokines, which promote inflammation. (Inflammation can be damaging when it's chronic, but it's crucially important for activating the immune cells in case of infection.) The omentum, an apron-like sheet of fat that hangs in front of the abdominal organs, is dotted with clumps of immune cells that act as hall monitors for the abdominal cavity, sampling the fluid between the organs for potential invaders, according to 2017 research.
Losing fat
In adulthood, the overall number of adipocytes stays stable, according to a 2008 paper in the journal Nature. Most weight loss and weight gain comes not from losing or gaining adipocytes, but from those cells expanding and shrinking as the energy inside is stored or burned. Adipocytes do gradually die off and get replaced, according to that study. The median turnover for fat cells is about 8.4 percent a year, with half of the fat cells in the body replaced every 8.3 years.
One of the biggest misconceptions about fat, according to Meerman, is that lost fat is literally burned off as energy.
"What really happens is that all of the atoms in fat combine with oxygen atoms to form carbon dioxide and water," he said. "Lots of energy is released by this process, but not one atom is destroyed or converted to energy."
The water from this process is excreted through urine, feces and sweat, Meerman reported in a 2014 British Medical Journal paper. The carbon dioxide is exhaled through your lungs, making your respiratory system your greatest fat-disposing tool.
Originally published on Live Science.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.