Salmonella Hides Its Tail to Stay Invisible to Immune System
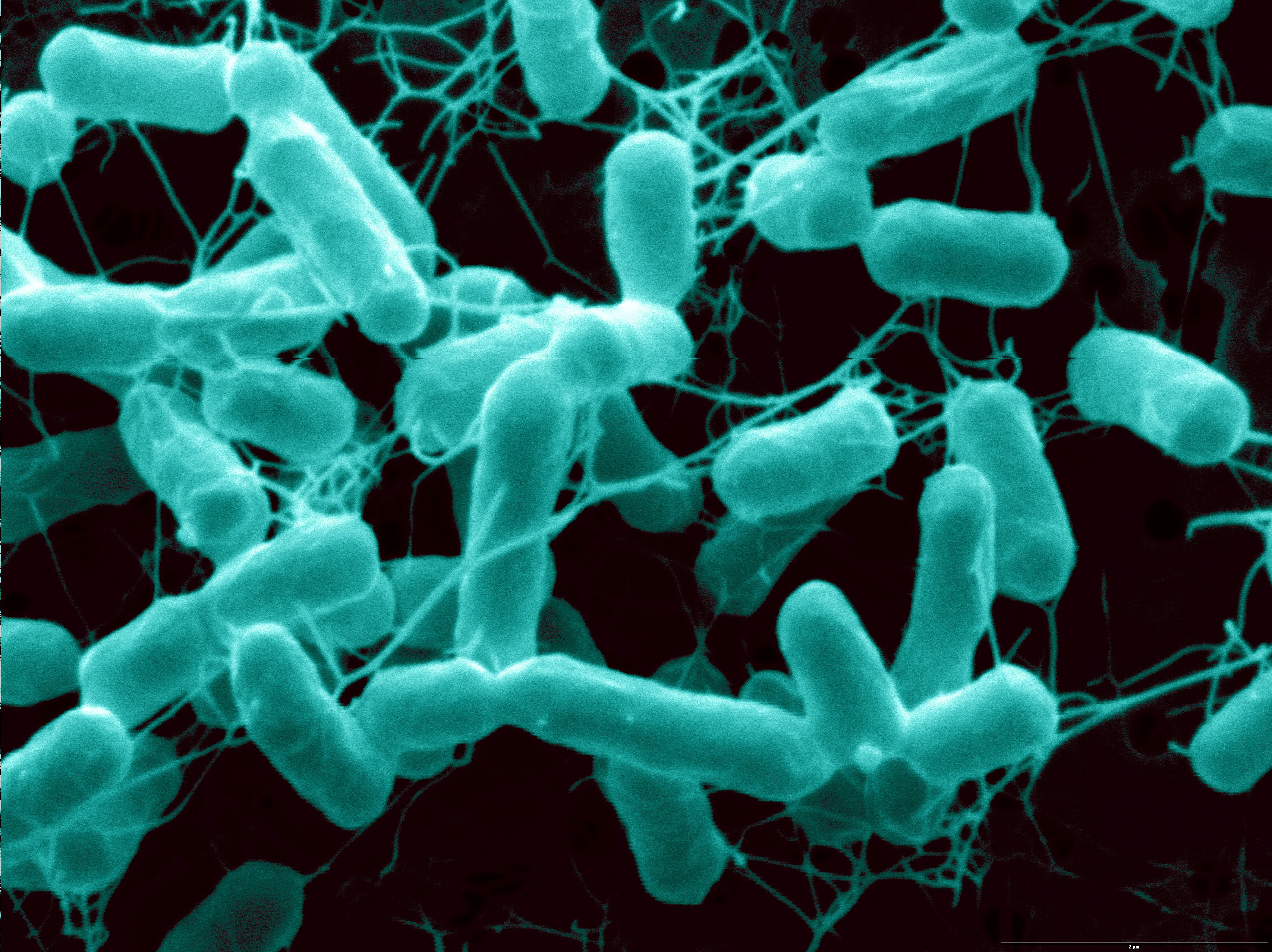
You'd be hard-pressed to find someone to say something good about Salmonella, a pervasive family of bacteria that sickens more than a million people each year in the United States.
But as bad as Salmonella's reputation is, the bug is certainly good at something: infecting us and causing misery. And now, scientists have discovered part of the reason why the bacteria are so talented at this: They've learned how to, quite literally, hide their tails and avoid detection by the immune system. And the discovery of that method is a good thing for us, because it may give scientists a new way to target and fight the bacteria. [Tiny & Nasty: Images of Things That Make Us Sick]
In a new study, published today (Oct. 23) in the journal Cell Reports, researchers found a tricky property of Salmonella Typhimurium (STM), the subspecies of this bacteria family that makes humans and other mammals sick. These bacteria can temporarily turn off their flagella, the tail-like appendages that whip to and fro, propelling the bacteria through the body.
"If you are bacteria [with] lots of flagella, it's like wearing a neon sign around your neck, basically alerting the immune system to your presence," said lead study author Brian Coombes, a professor in the Department of Biochemistry and Biomedical Sciences at McMaster University in Hamilton, Ontario. "Without that alert, it is a lot harder for the host to contain the bacteria's spread [and prevent them from going] to more cells."
In other words, by turning off that neon sign — or, in this case, those many neon propellers — the bacteria make it harder for your body's immune system to track down the invader and stop it.
Evading detection
Once STM bacteria invade a host cell — in this case, both mice and human cells in a laboratory setting — they use a genetic switch to stop their flagella activity, only to reactivate it when they leave to infect another cell, the researchers found. Coombes said he doesn't know of any other bacteria that behave this way, not even Salmonella bongori, the species that infects reptiles and other cold-blooded animals and has the same flagella genes.
"The loss of flagella has been reported in certain strains of bacteria that cause chronic infections of the gut and other mucosal surfaces … [but that] loss of flagella is permanent," Coombes told Live Science. "The process we identified [in Salmonella] is all controlled by regulation of the genes, so the bacteria doesn't have to delete them or mutate them. They just figured out how to turn them off at the right time. This allows them to turn [the genes] on … again later when the time is right."
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Salmonella, which is spread through contaminated food, causes about 1.2 million illnesses; 23,000 hospitalizations; and 450 deaths in the United States every year, according to the Centers for Disease Control and Prevention (CDC). And while the illness can, in most cases, be treated with antibiotics, doctors are concerned because some strains of the bacteria have become resistant to the drugs. Currently, a multidrug-resistant strain of Salmonella has contaminated raw chicken products in 29 states, leading to 21 hospitalizations, according to the CDC. [6 Superbugs to Watch Out For]
Disarming a threat
Dana Philpott, a professor of immunology at the University of Toronto, who was not involved with the study, said that the "findings highlight yet another way these pathogens hide from the host’s immune system."
But the newfound understanding of STM's invasion strategy may open up new ways to thwart the spread of the pathogen and perhaps other Salmonella types as well, Philpott told Live Science.
Indeed, the authors of the new study said they hope their findings will one day lead to non-antibiotic drugs that can fight even the resistant strains. Antibiotics directly kill bacteria, but bacteria can mutate in ways that make these drugs useless. A more effective approach may be to develop drugs that help the immune system kill the bacteria, Coombes said.
In the case of Salmonella, Coombes said he envisions a drug that prevents the bacteria from entering into their stealth mode, thus enabling the immune system to do its thing.
"Finding drugs that 'disarm' rather than outright kill bacteria, like antibiotics do, is an emerging area to help beat the antibiotic-resistance crisis," Coombes said. "Our immune systems are as close to the perfect natural antibiotic [as] you can find, and so by disarming bacteria of their virulence factors, the immune system regains the upper hand."
Follow Christopher Wanjek @wanjek for daily tweets on health and science with a humorous edge. Wanjek is the author of "Food at Work" and "Bad Medicine." His column, "Bad Medicine," appears regularly on Live Science.

Christopher Wanjek is a Live Science contributor and a health and science writer. He is the author of three science books: Spacefarers (2020), Food at Work (2005) and Bad Medicine (2003). His "Food at Work" book and project, concerning workers' health, safety and productivity, was commissioned by the U.N.'s International Labor Organization. For Live Science, Christopher covers public health, nutrition and biology, and he has written extensively for The Washington Post and Sky & Telescope among others, as well as for the NASA Goddard Space Flight Center, where he was a senior writer. Christopher holds a Master of Health degree from Harvard School of Public Health and a degree in journalism from Temple University.










