Breakthrough: Lab Lungs Live and Breathe
Scientists have built living, breathing lungs in the lab, a new advance that could one day help those in desperate need of these vital organs.
The researchers essentially took apart rat lungs and rebuilt them with new cells. After these new lungs were transplanted into live rats, for a short time they successfully exchanged oxygen and carbon dioxide and oxygenated the animals' blood, just as normal lungs do.
"This is an early step in the regeneration of entire lungs for larger animals and, eventually, for humans," said researcher Laura Niklason, a tissue engineer at Yale University in New Haven, Conn.
A new way to make lungs could prove lifesaving, as lung disease accounts for roughly 400,000 deaths each year in the United States alone. While hearts can regenerate and grow new cells throughout a person's life, lungs don't generally regenerate in the body beyond the cellular level. As such, currently the only way to replace damaged adult lung tissue is by performing lung transplantation, and donor lungs are in short supply. Moreover, lung transplantation is highly susceptible to organ rejection and infection, with only 10 to 20 percent of patients surviving the procedure after 10 years.
Growing lungs
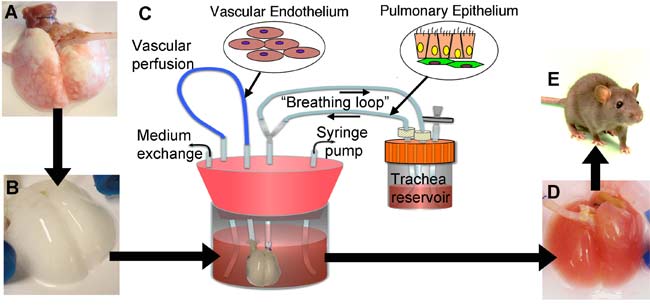
Niklason and her colleagues first took lungs from adult rats and delicately removed their existing cells with detergent. This left them with a scaffold of the connective tissue that retained the branching structure of the airways and blood vessels in the original lung, as well as its elasticity and other key mechanical properties. This strategy of using organs stripped of cells as scaffolds for engineered transplants is of growing interest, with successes recently seen with growing penis tissue, for instance, as well as hearts and maybe livers.
"It's this scaffolding that's the key," Niklason told LiveScience. "The problem with making synthetic scaffolds when it comes to lung tissue is that the architecture of the lung has such a highly complex structure — when you breathe in, your airway splits in two for each lung, and then each airway branches again and again for 23 generations of branching."
Get the world’s most fascinating discoveries delivered straight to your inbox.
"By the time you get to the bottom, you have millions and millions of tiny air sacs, a total area of 70 square meters (753 square feet)," she explained. "So as I thought about it, there's really no way I know of, or anyone knows of, to make a synthetic scaffolding to compare with that, so we didn't."
Next, the researchers injected a mixture of functional lung cells into this scaffold and soaked it all in a "bioreactor" designed to mimic fetal conditions. The bioreactors even make the engineering lungs within them breathe, squeezing back and forth to make fluid flow in and out of them.
"Fetuses breathe, actually — not air, but amniotic fluid," Niklason explained. "This intermittent breathing is actually pretty important for developing a functioning lung. We've studied a bunch of different breathing rates to see which work best, from once an hour to once a minute, and it seems that more frequent breathing results in better lung development."
In just a few days, the resulting lung tissue contained the kind of airways, blood vessels and air pockets seen in normal lungs. All these structures were populated with many of the correct kinds of cells and with the right types of mechanical characteristics. [images of lab lungs]
Future of lung regeneration
The scientists cautioned that a great deal more research is necessary to see if lungs could be made to sustain their function. When implanted into rats that had their left lungs removed, these engineered lungs functioned well for 45 minutes to two hours, but after roughly three hours, "we saw some evidence of clots forming in the lung," Niklason said.
The researchers suspect that when they seeded the scaffolds with cells, some blank spots might have been left behind where blood could pool to form clots. "That's something we can definitely improve on with more work," she noted.
Other research teams at the University of Minnesota and the University of Texas are also using mouse or rat lungs stripped of cells as scaffolds for growing lung tissue. However, Niklason and her colleagues are the first to implant such engineered tissue in living animals.
To prove these experiments could potentially work with human tissues, Niklason's team took segments of human lungs from a tissue bank and stripped out their cells to make scaffolds of them. Both human cancer cells and cells derived from human umbilical cord blood injected into these scaffolds successfully latched onto their surfaces, suggesting this method could prove effective.
Still, the kind of lung stem cells or so-called induced pluripotent stem cells needed to really make working human lungs are not available yet. Also, for this method to be useful to human patients, any cells used to reseed the scaffolds would have to come from the patient receiving the tissue transplant to avoid immune rejection.
"Regenerating lungs for patients is something I see as a 20- or 25-year prospect," Niklason said. "Still, I think we've put together a really solid platform for generating lungs for patients in the long-term."
The scientists detailed their findings online June 24 in the journal Science.



