How a Zebrafish Regrows a Fin
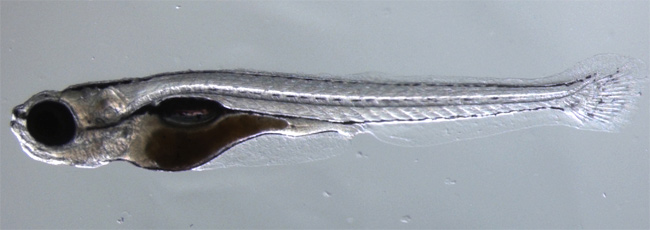
If a zebrafish loses a chunk of its tail fin, not to worry, it'll grow the fin back within a week. How this fish along with other cold-blooded animals, such as lizards, newts and frogs, can replace complex body parts with the ease of magicians has eluded scientists.
Now a study has revealed some of the genes responsible for the cellular pathways that let a zebrafish restore its tail fin.
Veterinary and medical scientists wonder if warm-blooded animals that evolved from these simpler creatures, might still have untapped regenerative powers hidden in their genes.
Supporting this notion, a tail fin is made up of several different types of cells arranged into an intricate structure, making it the fish version of an arm or leg. The results of the new study, therefore, could help doctors craft treatments for people whose hearts, spinal cords, eyes or arms and legs have been injured.
They know that as a human embryo matures, loads of cells await the command that directs each to become a certain type of cell with a particular function such as a heart-muscle cell. Once tissue formation begins, a red-light signal puts the brakes on cell growth before it gets out of control.
Dubbed Wnt/Beta-catenin signaling, cell-to-cell conversations control the fate of these as-yet undeveloped cells in the embryo.
A team of scientists found this same signaling pathway also sparks the regeneration and subsequent growth of cells that make up tail fins in zebrafish. Another signaling pathway under the control of the so-called Wnt5b protein then turns down these genes, impairs cell growth and inhibits fin regeneration.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Fish with a mutant Wnt5b protein re-grow lost tails quickly. Too much of a related protein also boosts cell growth in the regenerating fin. "We can actually increase the rate of regeneration by turning on these genes," said study team member Randall Moon of the University of Washington.
The same genes for orchestrating this growth and development are found in humans, and drugs exist that can regulate this pathway. So, Moon added, the findings could be used in figuring out ways for humans to re-build damaged organs some day.
Also, interfering with the "off-switch" genes could promote tissue regeneration in mammals such as humans, the scientists suggest in the Dec. 21 online edition of the scientific journal Development.
- Images: Freaky Fish
- Weirdest Science Stories of 2006
- Top 10 Animal Senses Humans Don't Have
- Strangest Animal Stories of 2006
Jeanna Bryner is managing editor of Scientific American. Previously she was editor in chief of Live Science and, prior to that, an editor at Scholastic's Science World magazine. Bryner has an English degree from Salisbury University, a master's degree in biogeochemistry and environmental sciences from the University of Maryland and a graduate science journalism degree from New York University. She has worked as a biologist in Florida, where she monitored wetlands and did field surveys for endangered species, including the gorgeous Florida Scrub Jay. She also received an ocean sciences journalism fellowship from the Woods Hole Oceanographic Institution. She is a firm believer that science is for everyone and that just about everything can be viewed through the lens of science.