Watch Supersonic Shock Waves Launch from a Bottle of Champagne
Scientists used a high-speed camera to record the moment the cork pops.
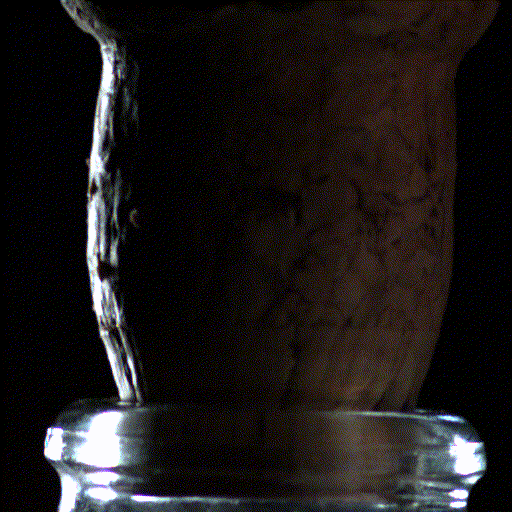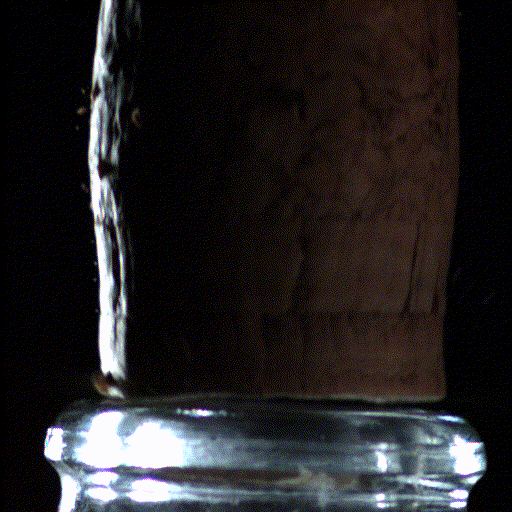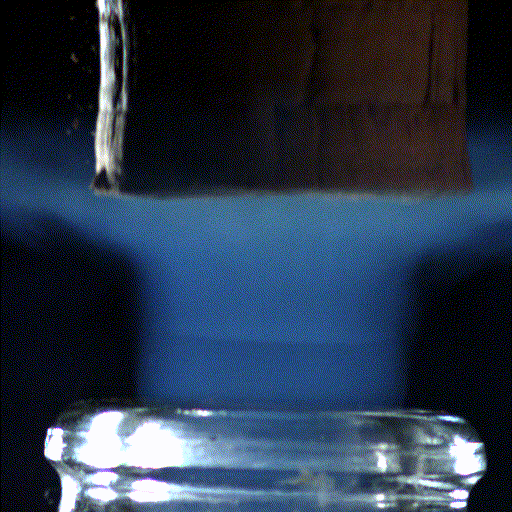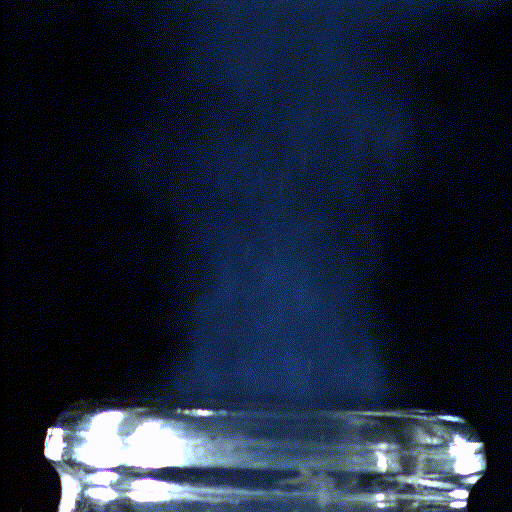
Popping open a bottle of bubbly creates shock waves like those in the supersonic exhaust of a fighter jet, according to a new study.
The split-second pop of a champagne cork is created by a quick escape of high-pressure gas long stuck in the bottle's neck. Now, a group of researchers has used high-speed photography to visualize the chemistry behind that iconic pop.
For the experiment, they acquired six champagne rosé bottles, two of which they stored at 30 degrees Celsius (86 degrees Fahrenheit) and two at 20 C (68 F) for three days. These bottles had been previously aged for 42 months, undergoing what's called "prise de mousse," a type of alcohol fermentation. During this process, yeast feeds on sugar to create carbon dioxide, giving champagne its fizz.
Related: Valentine's Bubbly: 9 Romantic Facts About Champagne
The researchers then used a high-speed camera to record the moment the corks popped. The high-speed camera was attached to a microphone that recorded the bang and triggered the camera to snap a series of photos.

Here's what the scientists saw: When the cork popped out of the bottle, it was violently shoved by rapidly expanding carbon dioxide and water vapor that had long been confined in the neck of the bottle. This sudden change in pressure caused the carbon dioxide and water vapor to cool down into ice crystals and condense into a fog that wafted out with the cork.
But to their surprise, the researchers found that within the first millisecond of the cork pop, this sudden drop in pressure inside the bottle led to visible shock waves, called "Mach disks." These Mach disks, which are also created in the exhaust of fighter jets, form because the escaping gas expands into the air extremely rapidly — at over twice the speed of sound. They vanish just as quickly, when the pressure in the bottle returns to normal.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

The formation of these Mach disks "was a big surprise," said lead author Gérard Liger-Belair, a professor of chemical physics at the University of Reims Champagne-Ardenne in France. "The physics [of Mach disks] was already known in aerospace engineering, but not [at] all in champagne science."
What's more, the researchers discovered that the bottles stored at room temperature created quite a different "pop" than those stored at hotter temperatures.
Because carbon dioxide is less soluble at higher temperatures, there is a greater amount of the gas sitting in the neck of the bottles stored at warmer temperatures. So the gas inside bottles stored at 30 C is under greater pressure than those stored at 20 C. When the cork in the 30 C bottle releases, the drop in pressure and temperature is greater than in the bottles stored at cooler temperatures.
The hotter bottle creates large ice crystals and, thanks to how those crystals scatter light, a greyish-white fog. The room-temperature bottle, meanwhile, creates smaller ice crystals, forming a bluer fog. "Hopefully, people will feel touched by the beautiful science hidden in a simple bottle of champagne or sparkling wine," Liger-Belair said.
The findings were published Sept. 20 in the journal Science Advances.
- Here's How 10 New Year's Eve Traditions Got Started
- Holiday Drinking: How 8 Common Medications Interact with Alcohol
- Supersonic! The 11 Fastest Military Airplanes
Originally published on Live Science.

Yasemin is a staff writer at Live Science, covering health, neuroscience and biology. Her work has appeared in Scientific American, Science and the San Jose Mercury News. She has a bachelor's degree in biomedical engineering from the University of Connecticut and a graduate certificate in science communication from the University of California, Santa Cruz.










