Scientists grow diamonds from scratch in 15 minutes thanks to groundbreaking new process
Scientists have used a new technique to synthesize diamonds at normal, atmospheric pressure and without a starter gem, which could make the precious gemstones easier to grow in the lab.

Scientists have used a new technique to synthesize diamonds at normal, atmospheric pressure and without a starter gem, which could make the precious gemstones much easier to grow in the lab.
Natural diamonds form in Earth's mantle, the molten zone buried hundreds of miles beneath the planet's surface. The process takes place under tremendous pressures of several gigapascals and scorching temperatures exceeding 2,700 degrees Fahrenheit (1,500 degrees Celsius).
Similar conditions are employed in the method currently used to synthesize 99% of all artificially created diamonds. Called high-pressure and high-temperature (HPHT) growth, this method uses these extreme settings to coax carbon dissolved in liquid metals, like iron, to convert it to diamond around a small seed, or starter diamond.
However, the high pressures and temperatures are difficult to produce and maintain. Plus, the components involved affect the diamonds' size, with the largest being about a cubic centimeter, or about as big as a blueberry. Besides, HPHT takes a fairly long time — a week or two — to produce even these tiny gems. Another method, called chemical vapor deposition, eliminates some requirements of HPHT, like high pressures. But others persist, like the need for seeds.
The new technique eliminates some drawbacks of both synthesis processes. A team led by Rodney Ruoff, a physical chemist at the Institute for Basic Science in South Korea, published their findings April 24 in the journal Nature.
Related: Scientists may have pinpointed the true origin of the Hope Diamond and other pristine gemstones
The diamond crucible
The novel method was a long time in the making. "For over a decade I have been thinking about new ways to grow diamonds, as I thought it might be possible to achieve this in what might be unexpected (per 'conventional' thinking) ways," Ruoff told Live Science by email.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
To start out, the researchers used electrically heated gallium with a bit of silicon in a graphite crucible. Gallium may seem like an esoteric element, but it was selected because a previous, unrelated study showed that it could catalyze the formation of graphene from methane. Graphene, like diamond, is pure carbon, but it contains the atoms in one layer rather than in the gemstone's tetrahedral orientation.
The researchers housed the crucible in a home-built chamber maintained at sea-level atmospheric pressure, through which superhot, carbon-rich methane gas could be flushed. Designed by co-author Won Kyung Seong, also of the Institute for Basic Science, this 2.4-gallon (9 liters) chamber could be readied for experimentation in just 15 minutes, allowing the team to rapidly undertake runs with different concentrations of metals and gases.
Through such tweaking, the researchers figured that a gallium-nickel-iron mixture — coupled with a pinch of silicon — was optimal for catalyzing the growth of diamonds. Indeed, with this blend, the team obtained diamonds from the crucible's base after just 15 minutes. Within two and a half hours, a more complete diamond film formed. Spectroscopic analyses showed that this film was largely pure but contained a few silicon atoms.
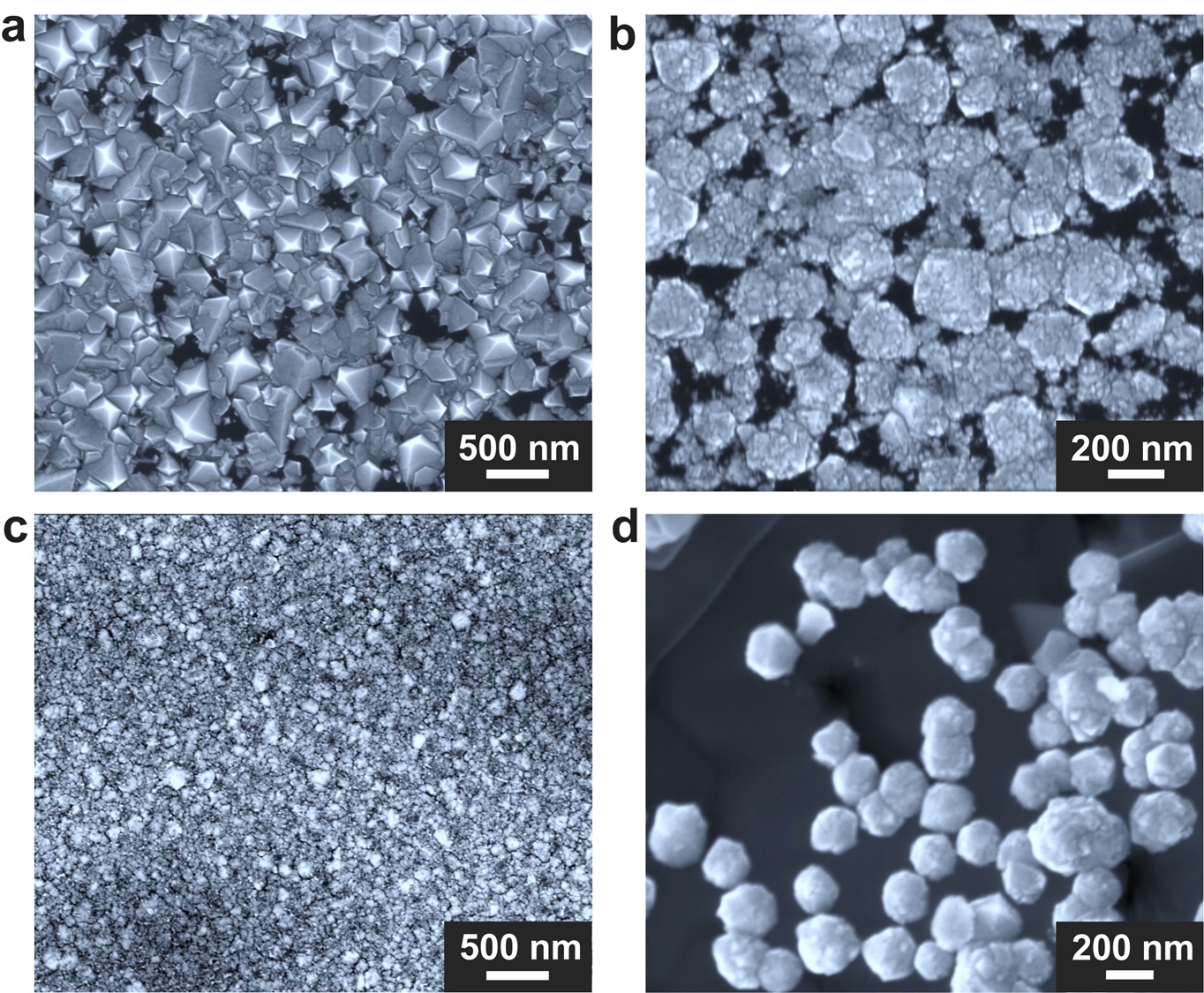
The minutiae of the mechanism that formed the diamonds are still largely murky, but the researchers think a temperature drop drives carbon from the methane toward the crucible's center, where it coalesces into diamond. Plus, without silicon, no diamonds form, so the researchers think it may act as a seed for the carbon to crystallize around.
However, the new method has its own challenges. One problem is that the diamonds grown with this technique are tiny; the largest ones are hundreds of thousands of times smaller than the ones grown with HPHT. That makes them too small to be used as jewels.
Other potential uses — for example, in more technological applications like polishing and drilling — for the diamonds synthesized with the new technique are unclear. However, because the process involves low pressure, Ruoff said, it might significantly scale up diamond synthesis.
"In about a year or two, the world might have a clearer picture of things like possible commercial impact," he added.

Deepa Jain is a freelance science writer from Bengaluru, India. Her educational background consists of a master's degree in biology from the Indian Institute of Science, Bengaluru, and an almost-completed bachelor's degree in archaeology from the University of Leicester, UK. She enjoys writing about astronomy, the natural world and archaeology.









