Coronavirus spike protein morphs into 10 different shapes to invade cells
These changes exposes more surfaces to potentially target with therapeutics.

The novel coronavirus uses its "spike proteins" to latch onto and invade human cells. But to do so, the spikes morph into at least 10 different shapes, according to a new study.
At the start of the pandemic, scientists rapidly identified the structure of the spike protein, paving the way to target it with vaccines and other drugs. But there's still so much scientists don't know about the interaction between the spike protein and the "doorknob" on the outsides of human cells — called the ACE2 protein. For instance, they aren't sure what intermediate steps the protein takes to kickstart the process of fusing to, and then opening the cell, ultimately dumping viral material into the cell.
"The spike protein is the focus of so much research at the minute," said co-lead author Donald Benton, a postdoctoral research fellow at the Francis Crick Institute's Structural Biology of Disease Processes Laboratory in the United Kingdom. Understanding how it functions "is very important because it's the target of most of the vaccination attempts and a lot of diagnostic work as well."
Related: Coronavirus live updates
To understand the process of infection, Benton and his team mixed human ACE2 proteins with spike proteins in the lab. They then used a very cold liquid ethane to rapidly freeze the proteins such that they became "suspended in a special form of ice," Benton told Live Science. They then put these samples under a cryo-electron microscope and obtained tens of thousands of high-resolution images of the spike proteins frozen at different stages of binding to the ACE2 receptors.
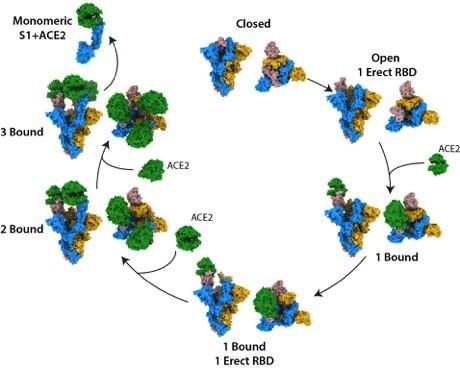
They found that the spike protein undergoes shape changes as it binds to the ACE2 receptor. After the spike protein first binds, its structure becomes more open to allow for more binding (imagine how much easier it would be to hug someone if they opened up their arms). The spike protein eventually binds to ACE2 at all three of its binding sites, revealing it's "central core," according to a statement. This final structure likely allows the virus to fuse to cell membranes.
"It's a very complicated receptor binding process compared to most virus spike proteins," Benton said. "Flu and HIV have a more simple activation process." The coronavirus is covered in spike proteins, and it's likely only a small fraction of them go through these conformational changes, bind to human cells and infect them, Benton said.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
"We know that the spike can adopt all these states that we were talking about," said co-lead author Antoni Wrobel, who is also a postdoctoral research fellow at the Francis Crick Institute's Structural Biology of Disease Processes Laboratory. "But whether each of the spikes adopts all of them we can't say because we can see only kind of snapshots."
The spike protein is very quick to change. In the lab, the spike can morph into all of these different conformations in less than 60 seconds, Wrobel told Live Science. But "this will be very different in a real infection; everything will be slower because the receptor will be stuck on the surface of a cell so you have to allow time for the virus to diffuse to this receptor," Benton said.
Why does the spike protein go through this many conformational changes to infect a cell? It "may be a way of the virus protecting itself from recognition by antibodies," Benton said. When the spike protein is in its closed states, it hides the site that binds with the receptor, maybe to avoid antibodies coming in and binding to that site instead, he said.
But "it's very hard to know," Wrobel said. In any case, this research reveals more surfaces on the spike protein that are exposed during infection — as different shapes reveal surfaces once thought hidden. Researchers can then potentially develop vaccines to target these surfaces. "We can then start to think about therapeutics that would fit somewhere either in the receptor surface or somewhere in the spike itself that then act as drugs," Wrobel told Live Science.
Wrobel and Benton hope to figure out why the coronavirus goes through so many conformational changes, how that compares to other coronaviruses and if these changes might help explain why this new virus spreads so easily.
The findings were published Sept. 17 in the journal Nature.
Originally published on Live Science.

Yasemin is a staff writer at Live Science, covering health, neuroscience and biology. Her work has appeared in Scientific American, Science and the San Jose Mercury News. She has a bachelor's degree in biomedical engineering from the University of Connecticut and a graduate certificate in science communication from the University of California, Santa Cruz.










