Quick guide: Most widely used COVID-19 vaccines and how they work
Here's how the top five COVID-19 vaccines work.

Editor's note: This article was last updated on March 22, 2022, by Live Science staff writer Nicoletta Lanese.
Dozens of coronavirus vaccines entered clinical trials during 2020, and now, more than 20 different shots are being administered to people around the world. Out of the five most widely used COVID-19 vaccines, three are cleared for use in the United States.
Here's a guide to how those top five vaccines work, their common side effects and how well the shots protect against SARS-CoV-2, the virus that causes COVID-19:
Pfizer-BioNTech
As of March 2022, the COVID-19 vaccine developed by Pfizer and German biotechnology company BioNTech is in use in 156 countries, including the U.S., according to The New York Times coronavirus vaccination tracker.
The vaccine was the first to be fully approved by the U.S. Food and Drug Administration (FDA), according to a statement from the agency. Full approval was granted on Aug. 23, 2021, roughly seven months after the shot had first been authorized for emergency use in the U.S.
The full approval allows the vaccine to be used in individuals ages 16 and older; meanwhile, the vaccine can be given to children ages five to 15 under an emergency use authorization, as it's yet to be fully approved for this age group, according to the Centers for Disease Control and Prevention (CDC).
Related: 11 surprising facts about the immune system
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
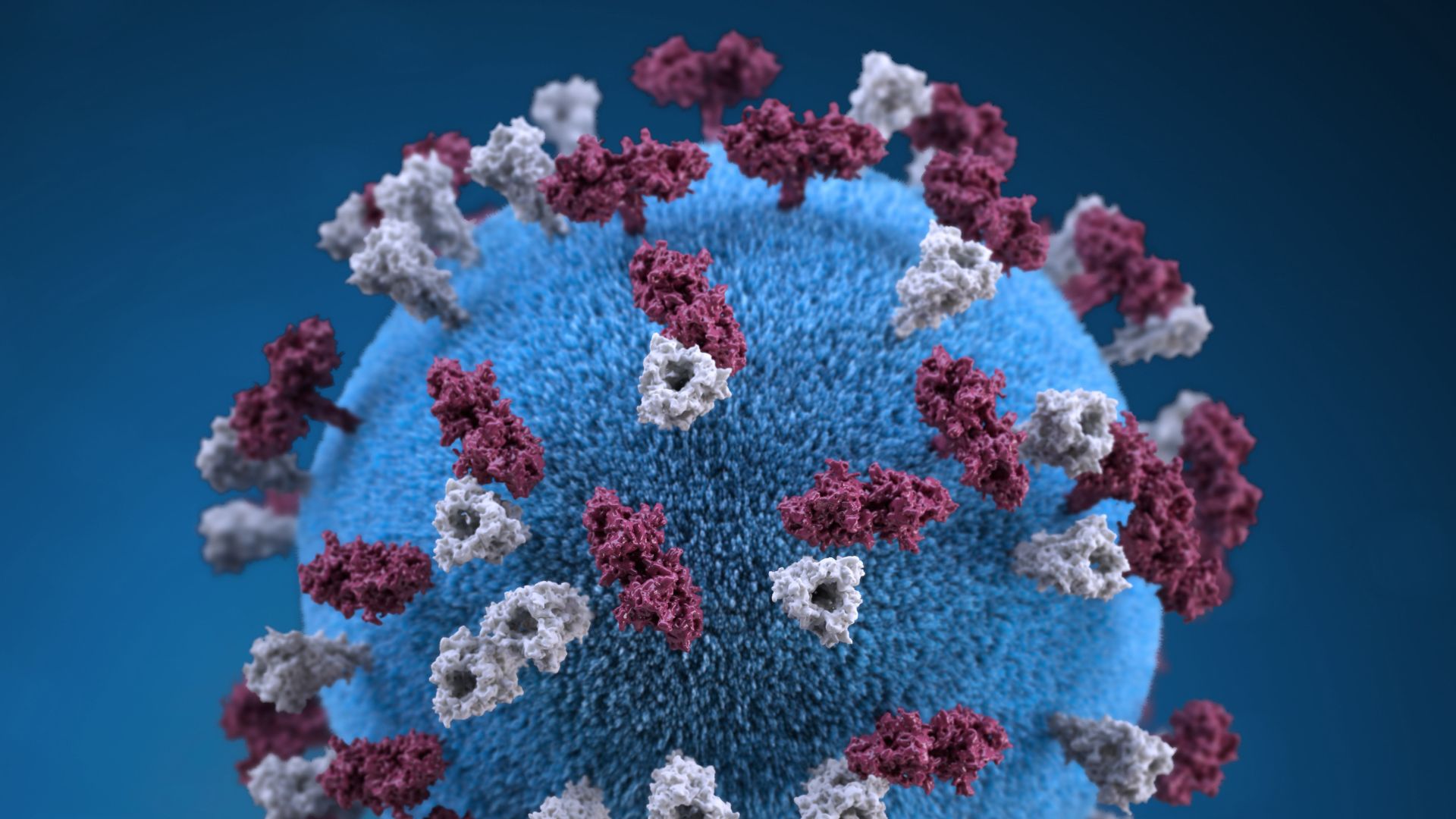
The Pfizer-BioNTech vaccine uses a molecule called messenger RNA (mRNA) as its base, the CDC notes. A molecular cousin of DNA, mRNA contains instructions to build specific proteins, and in this case, the mRNA in the vaccine codes for the coronavirus spike protein.
To build the vaccine, scientists place the mRNA inside a small bubble of fat, called a lipid nanoparticle; the shot also contains several salts and sugars, to help keep the vaccine's ingredients stable while it's manufactured, frozen, shipped and stored. (The Pfizer-BioNTech vaccine must be stored at minus 94 F (minus 70 C) to remain viable, according to The New York Times.)
Once injected into the body, the vaccine instructs human cells to build the spike protein, and the immune system learns to recognize and attack it, according to the CDC.
The vaccine is administered in two doses given 21 days apart. In the U.S., everyone aged 12 and older is now recommended to get a booster shot at least five months after completing their Pfizer-BioNTech primary series. Individuals ages 12 to 17 can only get a Pfizer-BioNTech booster, but older people can get either Pfizer-BioNTech or Moderna, the CDC notes. (The Johnson & Johnson vaccine is also available as a booster, but Pfizer-BioNTech or Moderna would be recommended, the CDC states.)
Common side effects include pain, redness and swelling at the injection site; tiredness; headache; muscle pain; chills; fever; and nausea. Rarely, inflammation of the heart muscle (myocarditis) and inflammation of the saclike membrane surrounding the heart (pericarditis) have been reported in teens and young adults who received the shot.
"These reports are rare, and the known and potential benefits of COVID-19 vaccination outweigh the known and potential risks, including the possible risk of myocarditis or pericarditis," the CDC notes. "The inflammation, in most cases, gets better on its own without treatment," according to Yale Medicine.
Late-stage clinical trials found that the vaccine was 95% effective at preventing laboratory-confirmed COVID-19 infections in people ages 16 and older, according to a report in the CDC journal Morbidity and Mortality Weekly Report (MMWR) published in December 2020. Later clinical trials suggested that the shots were similarly effective in children ages 5 to 15, according to the CDC.
That said, there's been mixed data on whether the shot offers the same level of protection against infection with omicron to children ages 5 to 11 as it does to older children and adults, STAT reported. Real-world data from New York state hinted that the shots provide less protection to the youngest age group, potentially because those children receive a smaller dose of vaccine than older teens and adults. However, new data from 10 states suggests that the vaccine is similarly protective in both groups, regardless of this dosing difference.
In general, data suggest that two doses of the vaccine provide about 70% protection against hospitalization and 33% protection against infection with the omicron variant, Healthline reported. A booster dose bolsters this protection, as early data suggest it's 70% to 75% effective against symptomatic infections and about 80% to 90% effective against severe disease.
Moderna
The COVID-19 vaccine developed by U.S. biotech company Moderna and the National Institute of Allergy and Infectious Diseases is now being used in the U.S. and 87 other countries, according to The New York Times coronavirus vaccination tracker.
It received full FDA approval on Jan. 31, 2022, for individuals ages 18 and older, according to the CDC. The FDA originally authorized the vaccine for emergency use on Dec. 18, 2020.
Like the Pfizer-BioNTech vaccine, the Moderna shot uses mRNA as its base and is administered in two doses. However, those doses are given four weeks apart, rather than three. People ages 18 and older should get a booster shot at least five months after completing their Moderna primary series, the CDC now recommends. In most instances, these individuals are recommended to get either a Pfizer-BioNTech or Moderna booster, rather than the Johnson & Johnson vaccine.
The Moderna vaccine can be stored at minus 4 F (minus 20 C), rather than requiring deep-freezing like the Pfizer-BioNTech shots.
Common side effects include pain, redness and swelling at the injection site; tiredness; headache; muscle pain; chills; fever; and nausea. As with the Pfizer-BioNTech shots, rarely, young adults who get the Moderna shots have developed myocarditis or pericarditis. Again, the potential benefits of COVID-19 vaccination outweigh this small risk, the CDC states.
Clinical trials found that the vaccine was 94.1% effective at preventing laboratory-confirmed COVID-19 infection in people who received two doses. There's not much data on how the shots hold up against omicron, but early studies hint that a three-dose course of the vaccine (that is, the two initial shots, plus a booster) offers 88% to 99% protection against hospitalization from omicron, Healthline reported. The three-dose course was about 47% to 71% protective against symptomatic omicron infections in healthy adults, although again, these estimates rely on preliminary data.
Related: 20 of the worst epidemics and pandemics in history
Oxford-AstraZeneca
The COVID-19 vaccine developed by the University of Oxford and pharmaceutical company AstraZeneca is now being used in 182 countries but not in the U.S., according to The New York Times coronavirus vaccination tracker.
The vaccine contains a modified version of an adenovirus, which is a type of virus that causes the common cold; specifically, the virus used in the AstraZeneca vaccine naturally infects chimpanzees, according to the Vaccine Knowledge Project, an informational site managed by an academic research group in the Department of Paediatrics at the University of Oxford.
Related: 5 dangerous myths about vaccines
Scientists modified the adenovirus so that it cannot infect human cells. Instead, the virus acts as a vessel to carry a short stretch of DNA into the body. That DNA codes for the coronavirus spike protein, a pointed structure that the virus uses to enter and infect cells. Once inside the body, the vaccine enters human cells and delivers these spike protein genes, which the cells then use to build the spike protein itself. The presence of spike proteins then triggers an immune response that trains the immune system to recognize and destroy the spike.
The Oxford-AstraZeneca vaccine is administered in two doses, spaced four to 12 weeks apart, the Vaccine Knowledge Project notes. In the U.K., people are now recommended to get a booster shot if three or more months have elapsed since their second dose of the vaccine. This recommendation applies to individuals ages 16 and older, plus children ages 12 to 15 who are at high-risk for severe COVID-19 or who live with an immunocompromised person. Most people in the U.K. receive either a Pfizer-BioNTech or Moderna shot for their booster, but some may be offered Oxford-AstraZeneca if they can't receive the other shots due to known allergies to the vaccines' ingredients, for instance.
Common side effects include pain near the injection site, chills, fever, joint pain, muscle aches, fatigue and headache. These flu-like symptoms typically occur in the few days following the injection. Very rarely, people who received the Oxford-AstraZeneca vaccine developed blood clots and low platelet counts, Live Science previously reported.
A large, late-stage clinical trial found that the Oxford-AstraZeneca vaccine is about 76% effective at preventing symptomatic COVID-19 infections, AstraZeneca reported in March 2021. In the same trial, the shot showed 100% efficacy against severe disease and hospitalization. At the time those results were published, the alpha, beta and gamma coronavirus variants had recently been named variants of concern, but the report didn't break down whether the shots were more or less protective against different variants.
In October 2021, the company also released real-world data on the vaccine's effectiveness; this new data took new coronavirus variants, such as delta, into account. According to the AstraZeneca statement, two doses of the vaccine are about 92% effective against severe disease or hospitalization due to the delta variant and about 70% effective against symptomatic delta infections.
However, early data from the U.K. Health Security Agency suggested that the vaccine offered "significantly lower" protection against symptomatic infections due to omicron than those due to delta. However, after a booster shot, the vaccine's effectiveness against omicron rose to between about 70% and 75%.
The vaccine can be stored at normal refrigerator temperatures and is expected to last for at least six months when stored at 36 to 46 degrees Fahrenheit (2.2 to 7.7 degrees Celsius), according to The Conversation.
Johnson & Johnson
The COVID-19 vaccine developed by Johnson & Johnson's Janssen is used in the U.S. and 86 other countries, according to The New York Times coronavirus vaccination tracker.
In clinical trials, the vaccine was about 72% effective at preventing symptomatic COVID-19 in the U.S., but across all the countries included in the trials, the vaccine was only 66% effective, according to Yale Medicine. This difference in protection was attributed to the highly contagious variants circulating in some countries at the time. Data later gathered in South Africa suggest that the shot is about 67% effective against hospitalization and about 82% effective against fatal disease from the delta variant, and the shot offered similar protection against beta.
Early data hint that the Johnson & Johnson vaccine may offer a similar level of protection against omicron as the Pfizer-BioNTech and Moderna shots, The New York Times reported in March 2022. This conclusion was based on data from people who had not received booster shots but had completed their primary vaccine series.
In addition, early data suggest that two doses of the Johnson & Johnson vaccine — meaning the primary dose plus a booster — offer a similar level of protection against symptomatic and severe omicron infections as three doses of an mRNA vaccine, according to the Times.
The vaccine has not been fully approved by the FDA but is available under emergency use authorization, the agency's website notes.
The single-dose vaccine is available both as a primary vaccination dose for individuals ages 18 and older and as a booster dose for individuals ages 18 and older who completed their primary vaccination at least two months prior. In general, the CDC recommends that people who received the Johnson & Johnson vaccine for their primary vaccination seek a Pfizer-BioNTech or Moderna shot as their booster.
"In most situations, Pfizer-BioNTech or Moderna COVID-19 vaccines are preferred over the J&J/Janssen COVID-19 vaccine for primary and booster vaccination due to the risk of serious adverse events [linked to the J&J vaccine]," the CDC states.
The most common side effects of the vaccine are fairly mild, including redness and pain at the injection site; tiredness; headache; muscle pain; chills; fever; and nausea. However, "there is a plausible causal relationship" between the Johnson & Johnson shot and a rare but potentially fatal blood clotting disorder called thrombosis with thrombocytopenia syndrome, where people develop blood clots and low platelet counts. "It occurs at a rate of about 3.83 cases per million Janssen doses," the CDC notes.
In July 2021, the FDA also issued a warning that there may be a link between the vaccine and Guillain-Barré syndrome (GBS), a neurological disorder in which the body’s immune system damages nerve cells. The available evidence hinted that there may be an increased risk of GBS after vaccination, but it was insufficient to establish a causal relationship, the FDA noted.
Like the Oxford-AstraZeneca vaccine, the Johnson & Johnson vaccine contains a modified adenovirus, which is filled with snippets of DNA, according to Nebraska Medicine. This particular adenovirus, called Ad26, has been modified such that it cannot replicate in cells and cause infection. Instead, the virus carries genetic instructions to build the coronavirus spike protein; once inside the body, the vaccine directs human cells to build the spike, which then provokes an immune reaction.
Sinopharm-Beijing
Sinopharm, the state-owned China National Pharmaceutical Group, and the Beijing Institute of Biological Products developed a COVID-19 vaccine that is now in use in 88 countries but not the U.S., according to The New York Times coronavirus vaccination tracker.
Clinical trials suggested that the vaccine had an efficacy of 79% against symptomatic COVID-19 infection, The New York Times reported, and in May 2021, an analysis by the World Health Organization concluded that the vaccine had an efficacy of 78.1% against symptomatic infection. The vaccine showed about 79% efficacy against hospitalization in both analyses.
However, real-world data from Peru suggested that the two-dose vaccine was only 50.4% effective in preventing infections; this data was collected as the lambda and gamma variants of the coronavirus were surging in the country, Reuters reported. And more recently, evidence emerged to suggest that the vaccine is much less protective against the delta and omicron variants, Quartz reported.
The vaccine is given in two doses spaced three to four weeks apart, according to Medical News Today. The WHO says that a booster shot can be given four to six months after this primary series. The booster shot can either be another dose of the Sinopharm vaccine or a different vaccine, although a study conducted in Bahrain suggested that getting a different vaccine might trigger a stronger immune response, the WHO notes.
Common side effects include headache, fatigue, fever, dizziness;, nausea, and redness and swelling at the injection site.
To make the vaccine, researchers took samples of the novel coronavirus from infected human patients and allowed the viruses to replicate in monkey kidney cells grown in bioreactor tanks, according to the Times. Then, they inactivated the viruses by applying a chemical called beta-propiolactone; once treated with this chemical, the viruses could no longer replicate but still carried all their characteristic proteins, including the spike.
The vaccine contains these inactivated viral particles and a substance called an adjuvant, which stimulates the immune system. Inside the body, specific immune cells gather up the dead viruses from the vaccine and display the viral proteins on their surfaces so that other immune cells can learn to recognize and attack the coronavirus.
Related: 11 (sometimes) deadly diseases that hopped across species
Bibliography
Almendral, A. (2021, December 29). How well do China’s vaccines work against omicron? Quartz. Retrieved March 22, 2022, from https://qz.com/2107603/are-sinovac-and-sinopharm-effective-against-omicron/
AstraZeneca. (2021, March 25). AZD1222 US Phase III primary analysis confirms safety and efficacy. AstraZeneca. Retrieved March 22, 2022, from https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/azd1222-us-phase-iii-primary-analysis-confirms-safety-and-efficacy.html
AstraZeneca. (2021, October). COVID-19 Vaccine AstraZeneca Real-World Evidence Summary. AstraZeneca. Retrieved March 22, 2022, from https://www.astrazeneca.com/content/dam/az/covid-19/media/factsheets/COVID-19_Vaccine_AstraZeneca_Real-World_Evidence_Summary.pdf
Branswell, H. (2022, February 28). Pfizer Covid vaccine is less effective in kids 5 to 11, study finds. STAT. Retrieved March 22, 2022, from https://www.statnews.com/2022/02/28/pfizer-covid-vaccine-kids-5-11/
Branswell, H. (2022, March 1). CDC data suggest Pfizer vaccine protection holds up in kids 5-11, raising questions on earlier study. STAT. Retrieved March 22, 2022, from https://www.statnews.com/2022/03/01/cdc-data-suggest-pfizer-vaccine-protection-holds-up-in-kids-5-11-raising-questions-on-earlier-study/
Centers for Disease Control and Prevention. (2022, February 1). Moderna COVID-19 Vaccine (also known as Spikevax) Overview and Safety. Centers for Disease Control and Prevention. Retrieved March 22, 2022, from https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html
Centers for Disease Control and Prevention. (2022, February 22). Johnson & Johnson’s Janssen COVID-19 Vaccine Overview and Safety. Centers for Disease Control and Prevention. Retrieved March 22, 2022, from https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html#When-to-Consider-J&J
Centers for Disease Control and Prevention. (2022, February 4). Pfizer-BioNTech COVID-19 Vaccine (also known as COMIRNATY) Overview and Safety. Centers for Disease Control and Prevention. Retrieved March 22, 2022, from https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html
Corum, J., & Zimmer, C. (2021, August 4). How the Sinopharm Vaccine Works. The New York Times. Retrieved March 22, 2022, from https://www.nytimes.com/interactive/2020/health/sinopharm-covid-19-vaccine.html
Corum, J., & Zimmer, C. (2021, May 7). How the Pfizer-BioNTech Vaccine Works. The New York Times. Retrieved March 22, 2022, from https://www.nytimes.com/interactive/2020/health/pfizer-biontech-covid-19-vaccine.html
Holder, J. (2022, March 20). Tracking Coronavirus Vaccinations Around the World. The New York Times. Retrieved March 22, 2022, from https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html
Katella, K. (2021, December 20). You Got the J&J Vaccine: Should You Get the booster? Yale Medicine. Retrieved March 22, 2022, from https://www.yalemedicine.org/news/johnson-and-johnson-covid-booster
Katella, K. (2022, March 2). Comparing the COVID-19 Vaccines: How Are They Different? Yale Medicine. Retrieved March 22, 2022, from https://www.yalemedicine.org/news/covid-19-vaccine-comparison
Klein, N. P., et al. (2022, March 4). Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA Vaccination in Preventing COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Nonimmunocompromised Children and Adolescents Aged 5–17 Years — VISION Network, 10 States, April 2021–January 2022. Centers for Disease Control and Prevention. Retrieved March 22, 2022, from https://www.cdc.gov/mmwr/volumes/71/wr/mm7109e3.htm?s_cid=mm7109e3_w
Mandavilli, A. (2021, August 6). New data suggest J. & J. vaccine works against Delta and recipients don’t need a booster shot. The New York Times. Retrieved March 22, 2022, from https://www.nytimes.com/2021/08/06/science/johnson-delta-vaccine-booster.html
Mandavilli, A. (2022, March 15). As Virus Data Mounts, the J.&J. Vaccine Holds Its Own. The New York Times. Retrieved March 22, 2022, from https://www.nytimes.com/2022/03/15/health/covid-johnson-vaccine.html
Mishra, S. (2020, November 24). Oxford-AstraZeneca vaccine is cheaper than Pfizer’s and Moderna’s and doesn’t require supercold temperature. The Conversation. Retrieved March 22, 2022, from https://theconversation.com/oxford-astrazeneca-vaccine-is-cheaper-than-pfizers-and-modernas-and-doesnt-require-supercold-temperature-150697
Nebraska Medicine. (2021, August 12). How the Johnson & Johnson COVID-19 vaccine works. Nebraska Medicine. Retrieved March 22, 2022, from https://www.nebraskamed.com/COVID/how-the-johnson-johnson-covid-19-vaccine-works
Oliver, S. E., et al. (2021, January 1). The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine — United States, December 2020. Centers for Disease Control and Prevention. Retrieved March 22, 2022, from https://www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm?s_cid=mm695152e1_w
Oliver, S. E., Gargano, J. W., Marin, M., Wallace, M., Curran, K. G., Chamberland, M., McClung, N., Campos-Outcalt, D., Morgan, R. L., Mbaeyi, S., Romero, J. R., Talbot, H. K., Lee, G. M., Bell, B. P., & Dooling, K. (2020, December 18). The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine — United States, December 2020. Centers for Disease Control and Prevention. Retrieved March 22, 2022, from https://www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm?s_cid=mm6950e2_w
Pike, H. (2021, May 18). Sinopharm COVID-19 vaccine: Should you worry about the side effects? Medical News Today. Retrieved March 22, 2022, from https://www.medicalnewstoday.com/articles/sinopharm-covid-19-vaccine-should-you-worry-about-the-side-effects
Rochabrun, M., & Liu, R. (2021, August 13). Peru study finds Sinopharm COVID vaccine 50.4% effective against infections. Reuters. Retrieved March 22, 2022, from https://www.reuters.com/world/americas/peru-study-finds-sinopharm-covid-vaccine-504-effective-against-infections-2021-08-13/
SAGE Working Group on COVID-19 vaccines. (2021, April 29). Evidence Assessment: Sinopharm/BBIBP COVID-19 vaccine. World Health Organization. Retrieved March 22, 2022, from https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/2_sage29apr2021_critical-evidence_sinopharm.pdf?sfvrsn=3dfe32c1_5
Sakay, Y. N. (2022, March 14). By the Numbers: COVID-19 Vaccines and Omicron. Healthline. Retrieved March 22, 2022, from https://www.healthline.com/health-news/by-the-numbers-covid-19-vaccines-and-omicron#2-dose-Pfizer-vaccine-vs.-Omicron
Tseng, H. F., et al. (2022). Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nature Medicine. https://doi.org/10.1038/ s41591-022-01753-y
U.K. Health Security Agency. (2021, December 10). SARS-CoV-2 variants of concern and variants under investigation in England - Technical briefing 31. U.K. Health Security Agency. Retrieved March 22, 2022, from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1027511/Vaccine-surveillance-report-week-42.pdf
U.K. Health Security Agency. (2021, December 31). SARS-CoV-2 variants of concern and variants under investigation in England - Technical briefing: Update on hospitalisation and vaccine effectiveness for Omicron VOC-21NOV-01 (B.1.1.529) . U.K. Health Security Agency. Retrieved March 22, 2022, from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1027511/Vaccine-surveillance-report-week-42.pdf
U.S. Food and Drug Administration. (2020, December 11). FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. U.S. Food and Drug Administration. Retrieved March 22, 2022, from https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
U.S. Food and Drug Administration. (2020, December 18). FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine. U.S. Food and Drug Administration. Retrieved March 22, 2022, from https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
U.S. Food and Drug Administration. (2021, August 23). FDA Approves First COVID-19 Vaccine. U.S. Food and Drug Administration. Retrieved March 22, 2022, from https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
U.S. Food and Drug Administration. (2021, July 13). Coronavirus (COVID-19) Update: July 13, 2021. U.S. Food and Drug Administration. Retrieved March 22, 2022, from https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-july-13-2021
U.S. Food and Drug Administration. (2022, March 10). Janssen COVID-19 Vaccine. U.S. Food and Drug Administration. Retrieved March 22, 2022, from https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine
Vaccine Knowledge Project. (2022, February 24). COVID-19 vaccines. Vaccine Knowledge Project. Retrieved March 22, 2022, from https://vk.ovg.ox.ac.uk/vk/covid-19-vaccines
World Health Organization. (2022, March 15). The Sinopharm COVID-19 vaccine: What you need to know. World Health Organization. Retrieved March 22, 2022, from https://www.who.int/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know
Originally published on Live Science.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.