Double pig kidney transplant successfully performed in brain-dead patient
The experiment was intended to assess the safety of such transplants, prior to them being tested in clinical trials.
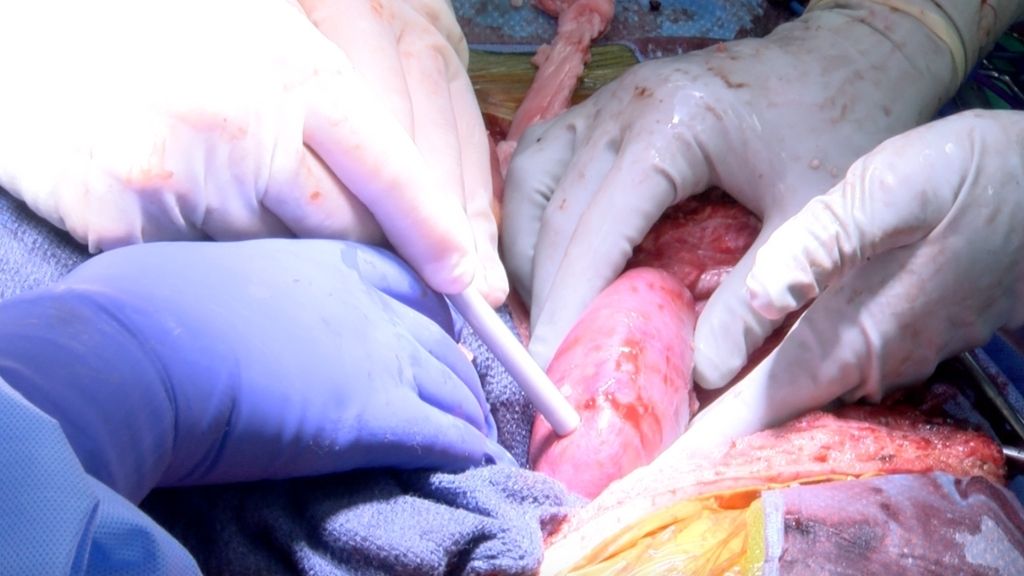
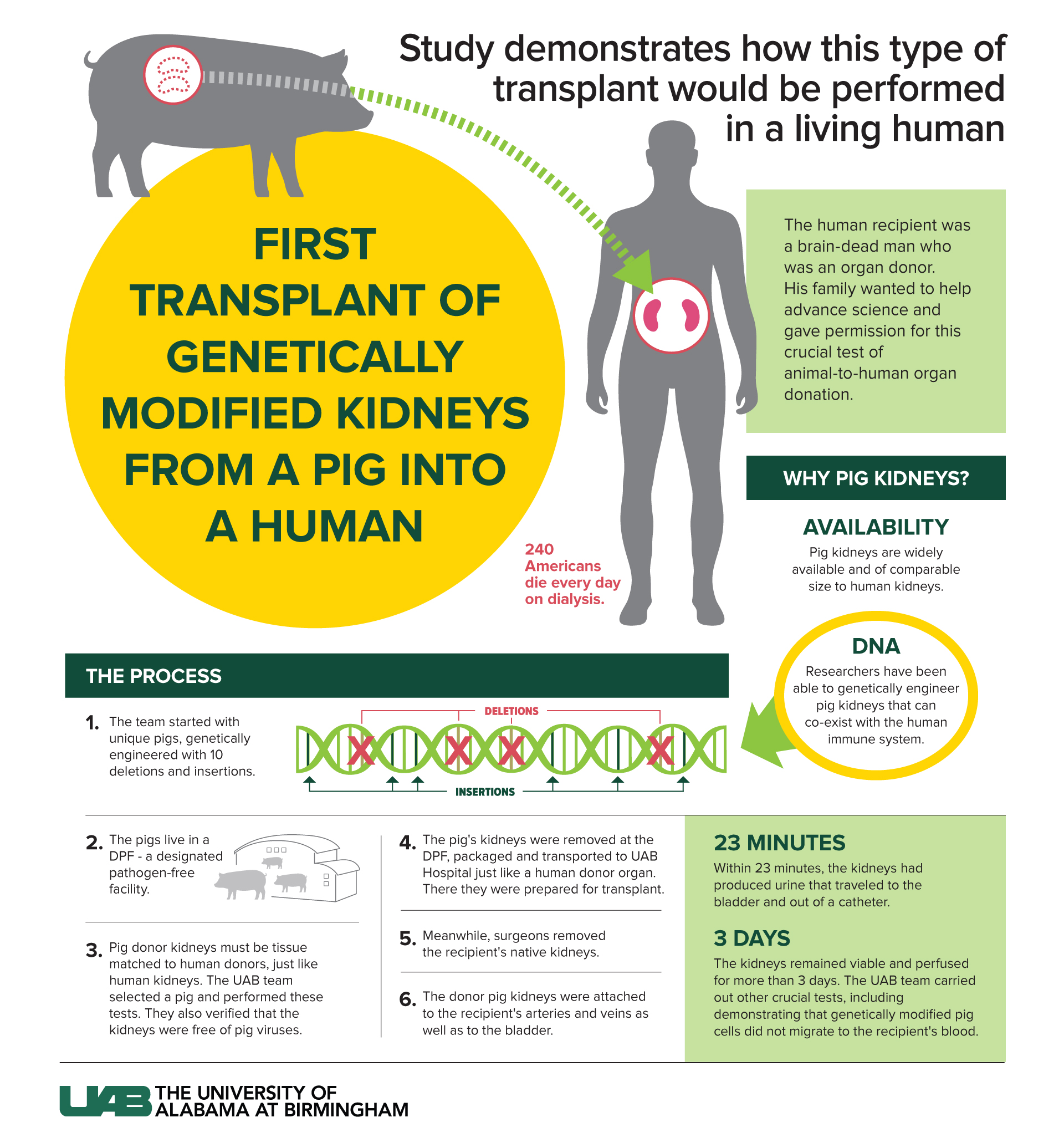
Scientists successfully transplanted two kidneys from a genetically modified pig into a human recipient and found that the organs produced urine and were not rejected during the days-long experiment.
The procedure was performed in a brain-dead patient who was a registered organ donor and whose family authorized the research, according to the new study, published Thursday (Jan. 20) in the American Journal of Transplantation. The research team intends to eventually transplant pig kidneys into living patients, in formal clinical trials — but first the team wanted to address some critical safety questions.
They tackled these questions in the organ recipient, monitoring him for any signs of transplant rejection, transmission of viruses from the pig donor or surgical complications that might be unique to the pig-to-human procedure. "This approach is founded on the premise that such questions must be answered before clinical trials of efficacy can be responsibly undertaken," the study authors wrote in their report.
Related: How long can organs stay outside the body before being transplanted?
In September 2021, doctors performed a similar experiment with a brain-dead patient at NYU Langone Health, during which they attached one genetically modified pig kidney to the patient, Live Science previously reported. The kidney functioned normally throughout the 54-hour study period, filtering waste from the blood and producing urine without any immediate signs of transplant rejection, the NYU team told news outlets. But the kidney remained outside the recipient's body for the entire experiment, hooked up to blood vessels in the upper leg.
In the new study, the researchers transplanted not one, but two pig kidneys inside a recipient's body, where kidneys would be placed during a conventional human-to-human transplantation, Dr. Jayme Locke, lead surgeon for the study and the director of the Comprehensive Transplant Institute in the University of Alabama at Birmingham (UAB) Department of Surgery, told Live Science in an email. From the procurement of the pig's kidneys to the surgery itself, the study followed the exact same procedure that the team will use in a future clinical trial, Locke said.
The kidneys used in the study came from a genetically modified pig developed by Revivicor, a subsidiary of United Therapeutics. (Several authors on the new paper are employees of Revivicor, and one is the company's chief scientific officer.) Earlier this month, doctors used a heart from another Revivicor pig to perform a first-of-its-kind heart transplant surgery, Live Science previously reported; the pig used for the heart transplant bore the same genetic modifications as the pig used in the new kidney transplant study, according to The New York Times.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Most of these genetic modifications are intended to reduce the risk of a transplant being rejected by the human body. For instance, the modified pigs lack three genes that each code for specific carbohydrates; in the human body, these carb molecules can set off an aggressive immune reaction. The donor pigs also lack a gene that codes for a specific growth hormone receptor, and without this receptor, the pigs' organs should stop growing once transplanted into a person.
Finally, the pigs carry six extra genes plucked from the human genome: four to help make each pig's organs appear more familiar to the human immune system and two to prevent the formation of blood clots.
After extracting the kidneys from their donor pig, the team inspected the organs. Overall, the pig kidneys closely resembled human kidneys, but differed in a few respects, the team noted.
For instance, the pig kidneys were softer to the touch; had a thinner capsule covering their outer surfaces; and the pig ureters — the ducts by which urine passes from the kidney to the bladder — were larger in diameter than typical human ureters. At this point, it's unclear whether these slight differences might affect the kidneys' function in a human, but "these observations underscored the need for meticulous handling and surgical technique," the study authors noted in their report.
The team prepared the human recipient for the transplant procedure by removing both his kidneys and providing immunosuppressive drugs, to reduce the risk of organ rejection. Then, after placing both pig kidneys into the recipient, the team monitored the organs for about three days.
In that time, the body didn't mount an immune response against the kidney, they observed. When a phenomenon called "hyperacute rejection" occurs, the body starts attacking a transplant organ soon after it is hooked up to the human circulatory system, once antibodies in the blood reach the organ. The donor pig had tested negative for porcine endogenous retroviruses — viruses that can hide in pig DNA and can infect human cells — and the team confirmed that there were also no signs of these viruses following the transplantation.
After transplantation, the right kidney initially showed "robust" urine production, while the left kidney produced much less urine, by comparison. The reason for this difference is unknown, but may be related to how each organ was initially procured from the donor pig, the authors noted. Compared to the right kidney, the left kidney spent more time at room temperature after being cut off from the pig's blood supply and before being placed on ice. More research is needed to know how such factors might impair the function of a pig organ in a human recipient, the authors wrote.
Although both kidneys produced urine, albeit in different quantities, neither organ filtered waste from the blood as a fully-functioning kidney would. The team found that the level of creatinine, a waste product of muscle cell function, in the blood did not decrease over time, and neither kidney excreted significant creatinine into the urine. It's unclear whether this dysfunction stemmed from damage to the kidneys, or was related to the physiological changes caused by brain death, the researchers noted.
"The brain death environment is quite hostile, making assessment of kidney function difficult," Locke said. Over the course of the experiment, the patient's organs began to fail, he developed abnormal blood clotting, and his blood also became more acidic due to a build-up of hydrogen ions. The researchers used various medications and infusions to counter these effects of brain death during the study, but even so, the effects might have undermined the pig kidneys' function, the authors wrote.
"This was not a surprising observation to us given that, even in human-to-human transplantation, kidneys from brain-dead donors often have delayed graft function, meaning that they often do not make urine for a week and take several more weeks to clear creatinine," Locke told Live Science.
Overall, the study suggests that, while many barriers to pig-to-human kidney transplants have been surmounted, many questions about the procedure remain unanswered, the authors wrote. Future studies in brain-dead individuals could provide some of the answers to these questions, while others may need to be investigated in non-human primates. And eventually, some questions will be addressed in clinical trials in living humans.
Locke and her colleagues are now going through the process of submitting a Investigational New Drug Application to the U.S. Food and Drug Administration; once authorized, this will allow the team to use the genetically modified pig kidneys in a clinical trial. They will also need to obtain approval for such a trial through UAB's institutional review board. "Both of these efforts are well under way," Locke said.
Originally published on Live Science.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.










