The moon is rusty, and it’s likely Earth's fault
No wonder the moon is inching away.

The moon is turning ever so slightly red, and it's likely Earth's fault. Our planet's atmosphere may be causing the moon to rust, new research finds.
Rust, also known as an iron oxide, is a reddish compound that forms when iron is exposed to water and oxygen. Rust is the result of a common chemical reaction for nails, gates, the Grand Canyon's red rocks — and even Mars. The Red Planet is nicknamed after its reddish hue that comes from the rust it acquired long ago when iron on its surface combined with oxygen and water, according to a statement from NASA's Jet Propulsion Laboratory (JPL) in Pasadena, California.
But not all celestial environments are optimal for rusting, especially our dry, atmosphere-free moon.
"It's very puzzling," study lead author Shuai Li, an assistant researcher at the University of Hawaii at Mānoa's Hawaii Institute of Geophysics and Planetology, said in the statement. "The Moon is a terrible environment for [rust] to form in."
Related: In photos: India's Chandrayaan-2 mission to the moon
Li was studying data from the JPL Moon Mineralogy Mapper,which was onboard the Indian Space research Organization's Chandrayaan-1 orbiter while it surveyed the moon in 2008, when he realized that the poles of the moon had very different compositions than the rest of it.
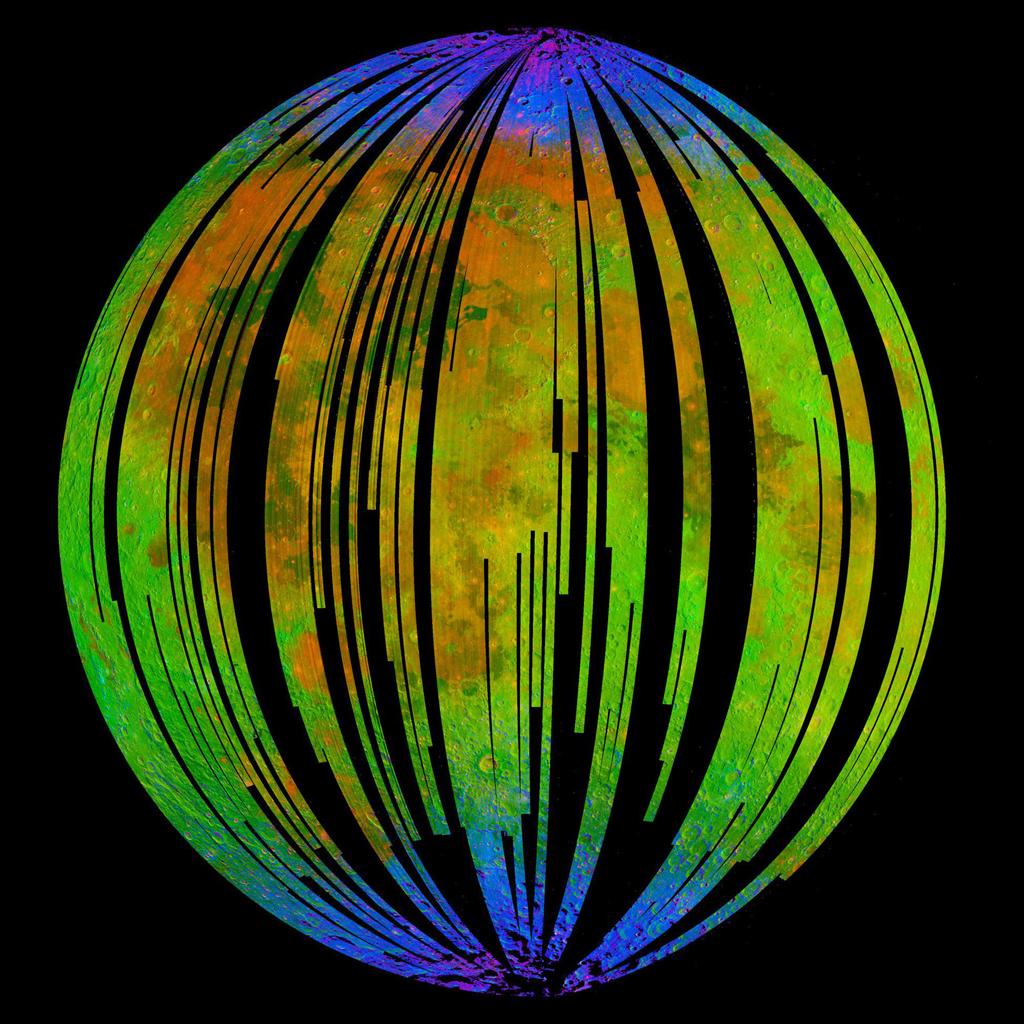
During its mission, the Moon Mineralogy Mapper detected spectra, or wavelengths of light reflected off various surfaces of the moon, to analyze its surface makeup. When Li focused on the poles, he found that the moon's polar surfaces had iron-rich rocks with spectral signatures that matched that of hematite. The mineral hematite, commonly found in Earth's surface, is a specific type of iron oxide, or rust, with the formula Fe2O3.
Get the world’s most fascinating discoveries delivered straight to your inbox.
"At first, I totally didn't believe it. It shouldn't exist based on the conditions present on the Moon," co-author Abigail Fraeman, a planetary geoscientist at JPL, said in the statement. "But since we discovered water on the Moon, people have been speculating that there could be a greater variety of minerals than we realize if that water had reacted with rocks."

What on Earth happened
For iron to turn rusty red, it needs what's called an oxidizer — a molecule such as oxygen that removes electrons from a material such as iron. But the sun's solar wind, a stream of charged particles that constantly hits the moon with hydrogen, has the opposite effect. Hydrogen is a reducer, or a molecule that donates electrons to other molecules. Without protection from this solar wind, such as the magnetic field that shields our planet from it, rust should not be able to form on the moon.
But it does, and the key might be our own planet.
The moon doesn't have an atmosphere of its own to provide sufficient amounts of oxygen, but it has trace amounts donated by Earth's atmosphere, according to the statement. This terrestrial oxygen travels to the moon along an elongated extension of the planet's magnetic field called a "magnetotail."
Earth's magnetotail can reach all the way to the near side of the moon, where more of the hematite was found, according to the statement. What's more, at every full moon, the magnetotail blocks 99% of solar wind from blasting the moon, drawing a temporary curtain over the lunar surface, allowing periods of time for rust to form. But there's still one extra ingredient that's needed for rust to form: water.
The moon is mostly devoid of water, save for frozen water found in lunar craters on the moon's far side — far from where most of the hematite was found. But the researchers propose that fast-moving dust particles that bombard the moon might free water molecules locked into the moon's surface layer, allowing the water to mix with the iron. These dust particles might even be carrying water molecules themselves, and their impact might create heat that could increase the oxidation rate, the researchers said.
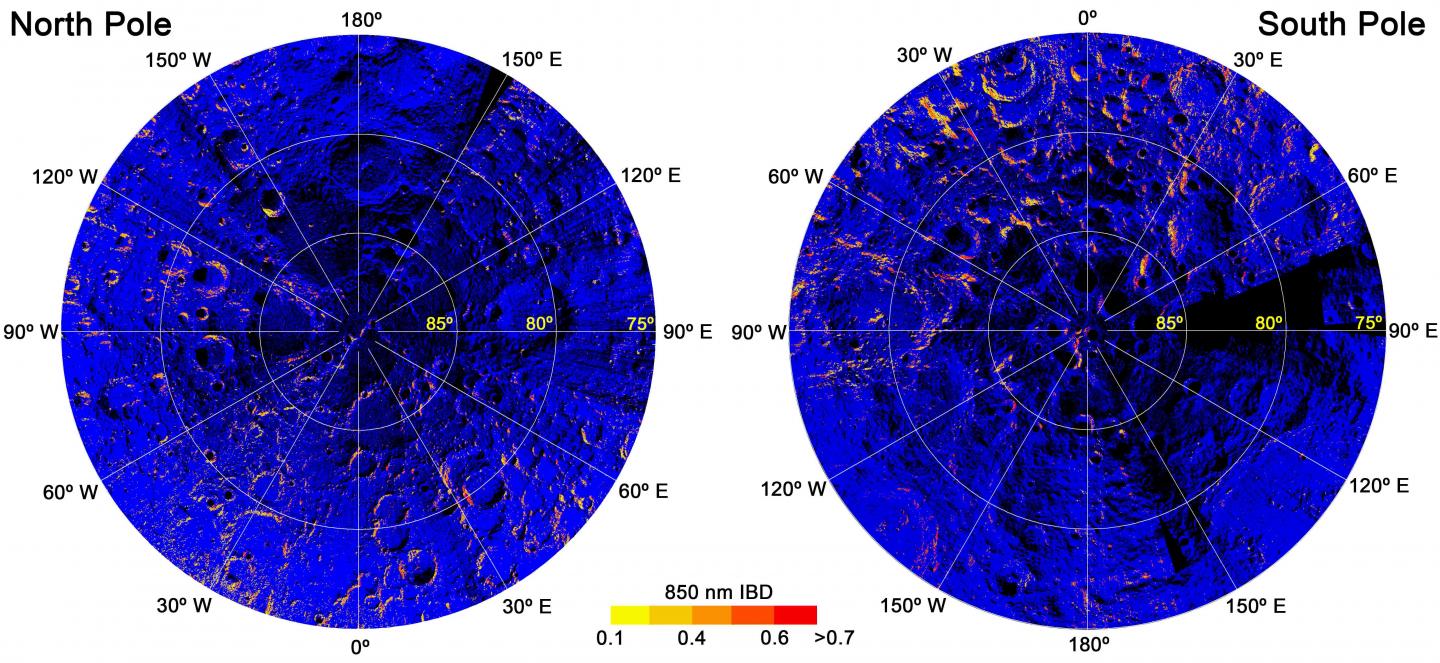
"This discovery will reshape our knowledge about the Moon's polar regions," Li said in a separate statement from the University of Hawaii. "Earth may have played an important role on the evolution of the Moon's surface."
However, these are still hypotheses and more data is needed to understand exactly why the moon is rusting. Even more surprising, small amounts of hematite have been found on the far side of the moon, which should be too far for Earth's oxygen to hitch a ride on the planet's magnetotail, according to the statement.
The findings were published on Sept. 2 in the journal Science Advances.
Originally published on Live Science.

Yasemin is a staff writer at Live Science, covering health, neuroscience and biology. Her work has appeared in Scientific American, Science and the San Jose Mercury News. She has a bachelor's degree in biomedical engineering from the University of Connecticut and a graduate certificate in science communication from the University of California, Santa Cruz.
