FDA to authorize 3rd COVID-19 vaccine dose for immunocompromised people
Around 2.7% of adults in the U.S. are immunocompromised.
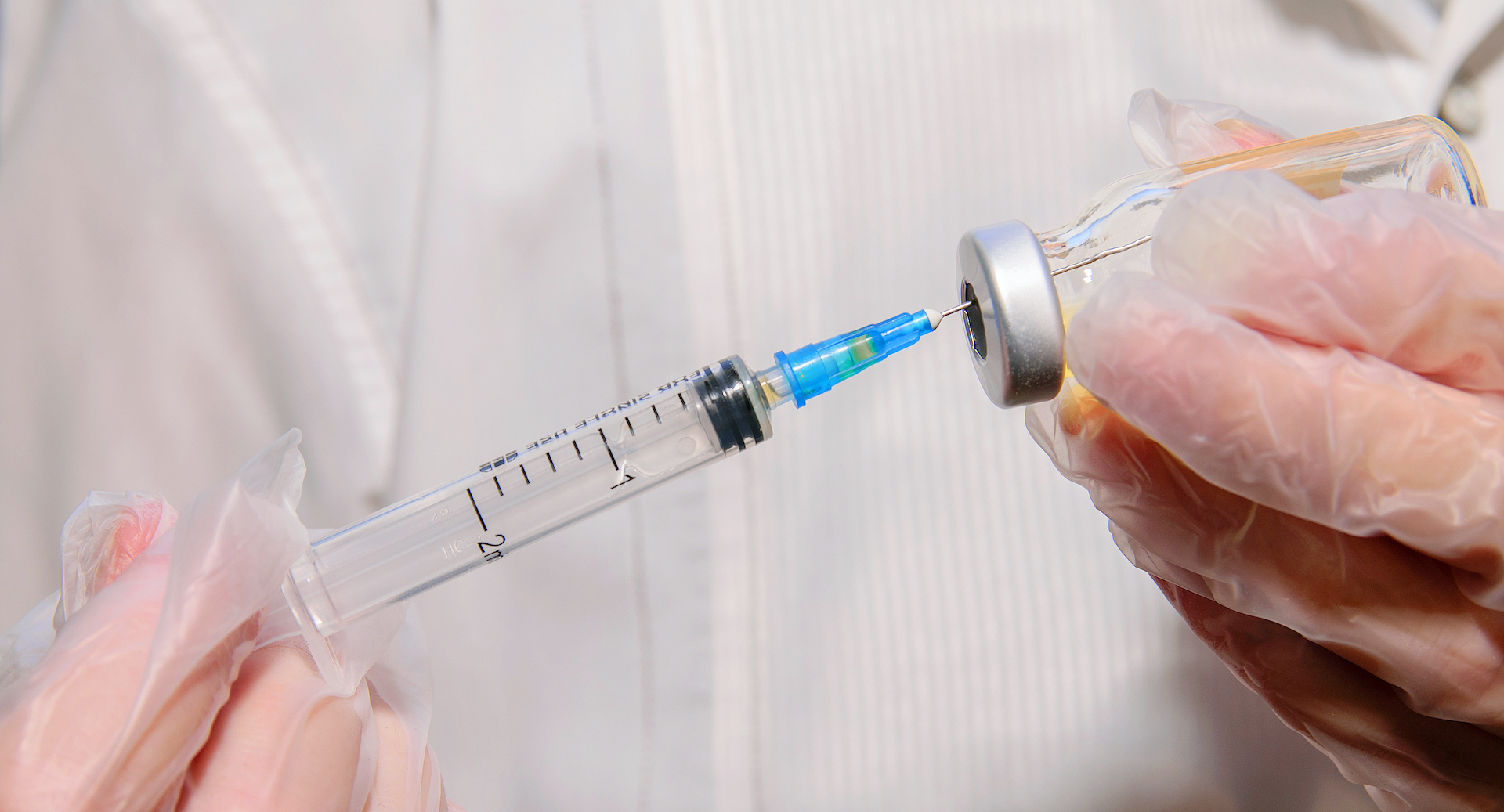
On Thursday (Aug. 12), the U.S. Food and Drug Administration (FDA) is planning to authorize a third vaccine dose for people with weakened immune systems, according to recent news reports.
The FDA will update the emergency use authorizations for the two mRNA vaccines — Pfizer/BioNTech and Moderna —to include a third dose for immunocompromised patients. That would be the first authorization of a third dose in the U.S., according to NBC News, which first reported the news.
Around 2.7% of the adult population in the U.S. are immunocompromised, according to meeting slides from an advisory committee to the Centers for Disease Control and Prevention (CDC) that met in July to discuss the supplemental doses.
Related: Coronavirus variants: Here's how the SARS-CoV-2 mutants stack up
Such patients may include those who have tumors, HIV, organ transplants or are being treated with immune system suppressing medications. But it's not yet clear which conditions will be included in the authorization for a third dose, according to NBC News.
Studies have suggested that people with weakened immune systems do not mount as strong a response against SARS-CoV-2 as healthy controls. In a small study of 30 organ transplant patients, 24 developed no antibodies against the coronavirus after receiving two doses of an mRNA vaccine and six only developed low levels of such antibodies, NBC News reported.
What's more, people who are immunocompromised are more likely to become severely ill from COVID-19, have a longer disease duration, more likely to transmit the virus to household contact and are at higher risk for a breakthrough infection, according to the advisory committee slides.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
But emerging data suggests that an additional dose of an mRNA vaccine improves antibody production and increases the proportion of people who respond to the vaccine, according to the slides. For example, several studies found that in immunocompromised patients who had no detectable antibody response to the first two doses of mRNA vaccines, between one-third and one-half developed a response to the third dose, according to the slides.
In the organ transplant study, two weeks after receiving a third dose of one of the authorized COVID-19 vaccines, eight out of the 24 patients who had no antibodies developed some antibodies and all six who had low levels showed an increase, according to NBC News.
After the FDA amends the authorization, the CDC advisory group will meet and vote whether to officially recommend the third dose for immunocompromised patients, according to NBC News.
If the advisory committee gives the OK, people who fall into the correct categories will be allowed to receive their third dose.
Originally published on Live Science.

Yasemin is a staff writer at Live Science, covering health, neuroscience and biology. Her work has appeared in Scientific American, Science and the San Jose Mercury News. She has a bachelor's degree in biomedical engineering from the University of Connecticut and a graduate certificate in science communication from the University of California, Santa Cruz.









