Healthy breast cells can look like invasive cancer, complicating early diagnosis
Scientists found that healthy women can carry genetic changes in their breast cells that are thought to be characteristic of invasive breast cancer.
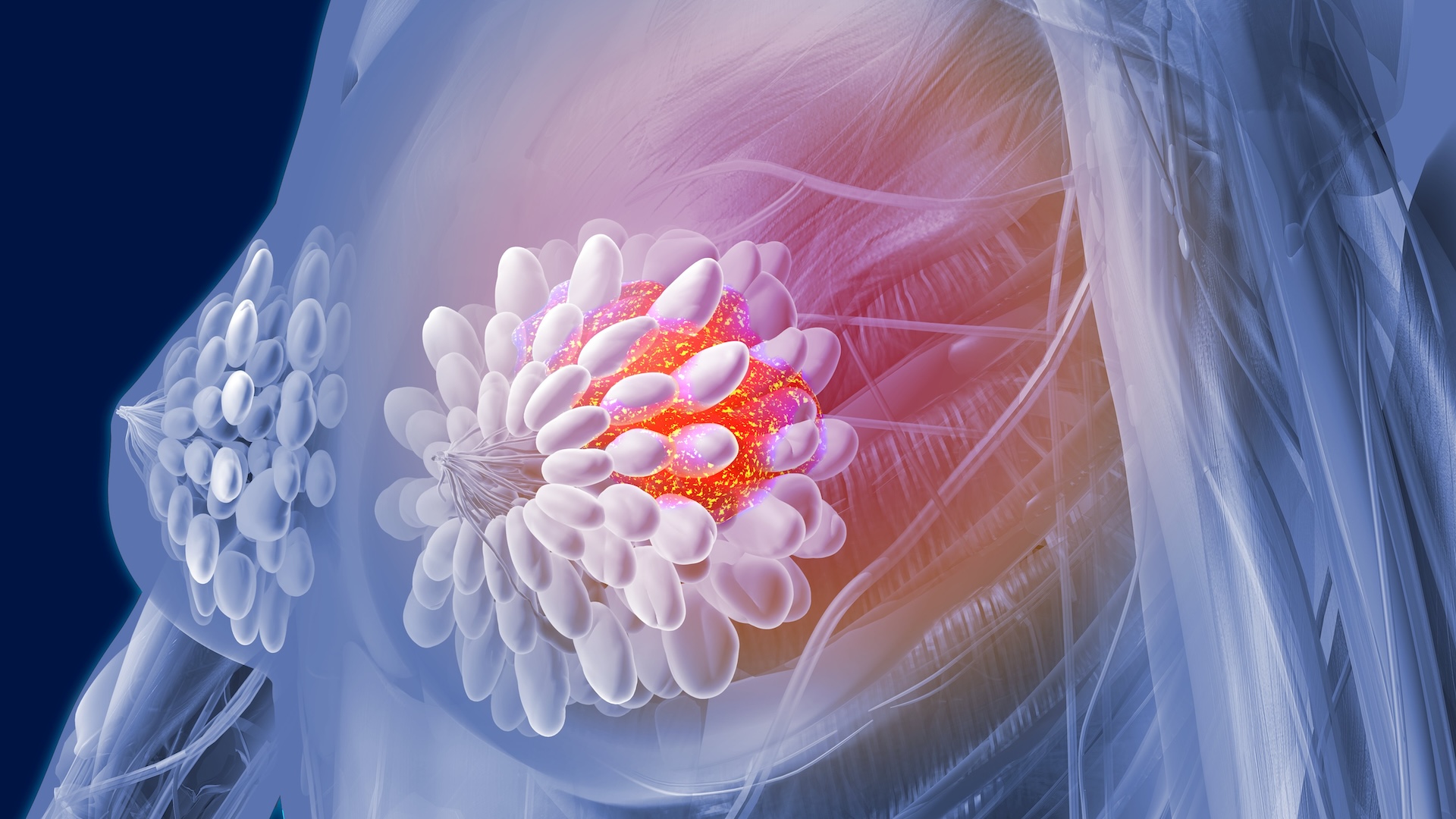
Most cancer cells have too many or too few chromosomes, distinguishing them from normal cells. But a new study shows that healthy breast tissues can also have cells with unusual copies of chromosomes.
The finding challenges how scientists classify cancerous cells and could influence how early breast cancer is identified in the future.
Each cell in the human body has 23 pairs of chromosomes — half inherited from the mother and half from the father. During cell division, each cell makes copies of these chromosomes to pass down to two daughter cells. But on rare occasions, such as during tumor development, this process may be derailed, resulting in an extra or missing copy — known as aneuploidy.
Related: Doctors no longer recommend 'self-checks' for breast cancer
Research has shown that about 9 in 10 solid tumors harbor some aneuploidy. However, aneuploidy in healthy cells was considered rare, and hence it was thought to be a valuable marker for screening for cancer. So scientists have begun developing screening methods that test for aneuploidy in patients’ blood and tissue samples. However, the approach has not been widely used in the clinic.
In the new study, published Nov. 20 in the journal Nature, scientists found that about 3% of the cells in the inner lining of a healthy breast, known as breast epithelial cells, are aneuploid. More than 80% of these aneuploid cells had changes in their DNA structure that may alter gene expression — how genes are switched on or off — and lead to diseases, such as invasive cancer.
"This was very unexpected," study co-author Nicholas Navin, a professor at the University of Texas' MD Anderson Cancer Center, told Live Science in an email. "We would have classified these cells as having invasive breast cancer based on these [DNA] aberrations."
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Navin and his team analyzed more than 83,000 breast epithelial cells from 49 healthy women who had undergone breast reduction surgery and did not have cancer. They profiled the genetic makeup of these women and then used another test, called the assay for transposase-accessible chromatin sequencing (ATAC-seq), to look for any genetic anomalies characteristic of an invasive cancer.
To their surprise, the researchers found that some healthy breast epithelial cells contained changes similar to ones seen in cancer cells. For example, one healthy woman had 70 cells with extra copies of chromosome 1 and 73, as well as missing copies of chromosome 16.
The most frequent chromosome changes in these healthy women were extra copies in chromosome 1, and losses of chromosomes 16, 10 and 22, which scientists consider the hallmarks of an invasive cancer. These changes are often associated with distinct types of cells in the milk ducts that lead to different breast cancers. For example, losses of chromosomes 16 and 22 are often associated with breast cancers that are brought on by levels of the hormone estrogen, also known as estrogen-receptor (ER)-positive breast cancer. A loss of chromosome 10, meanwhile, is associated with ER-negative breast cancer.
"We did not expect to find these chromosomal events in normal breast tissues," Navin said. The loss of chromosome 16 has been used to identify invasive breast cancer.
It's not clear yet whether the women in the study will develop breast cancer. Now, "the question is whether women with higher frequencies of aneuploid epithelial cells or CNA (copy number alteration) events are at higher risk for developing breast cancer," Navin said.
The discovery also led the scientists to speculate that distinct types of breast cancers could originate from certain healthy cells in the female breast.
Navin and his team are now investigating whether aneuploidy could be used to identify tissue in healthy women that are more likely to develop tumors.
Disclaimer
This article is for informational purposes only and is not meant to offer medical advice.

Kristel is a science writer based in the U.S. with a doctorate in chemistry from the University of New South Wales, Australia. She holds a master's degree in science communication from the University of California, Santa Cruz. Her work has appeared in Drug Discovery News, Science, Eos and Mongabay, among other outlets. She received the 2022 Eric and Wendy Schmidt Awards for Excellence in Science Communications.